
Source: Stem cell says
Pluripotent Stem Cells (PSC) are represented by Embryonic Stem Cells (ESC) and induced Pluripotent Stem Cells (iPSC). The isolation of human ESC from blastocyst embryos was first reported in 1998, and the success of human iPSC was first reported in 2007.
The discovery of pluripotent stem cells has revolutionized stem cell research and cell-based therapies, opening up a new era of regenerative medicine, specifically cell therapy to replace lost or damaged tissue. Pluripotent stem cells are increasingly being explored for cell therapy in a variety of diseases and injuries (e.g., Parkinson's, spinal cord injury, age-related macular degeneration, etc.). Regarding clinical application, the ethical problems and immune rejection problems of ESC are prominent, which makes iPSCs have a greater application prospect in the treatment of diseases.
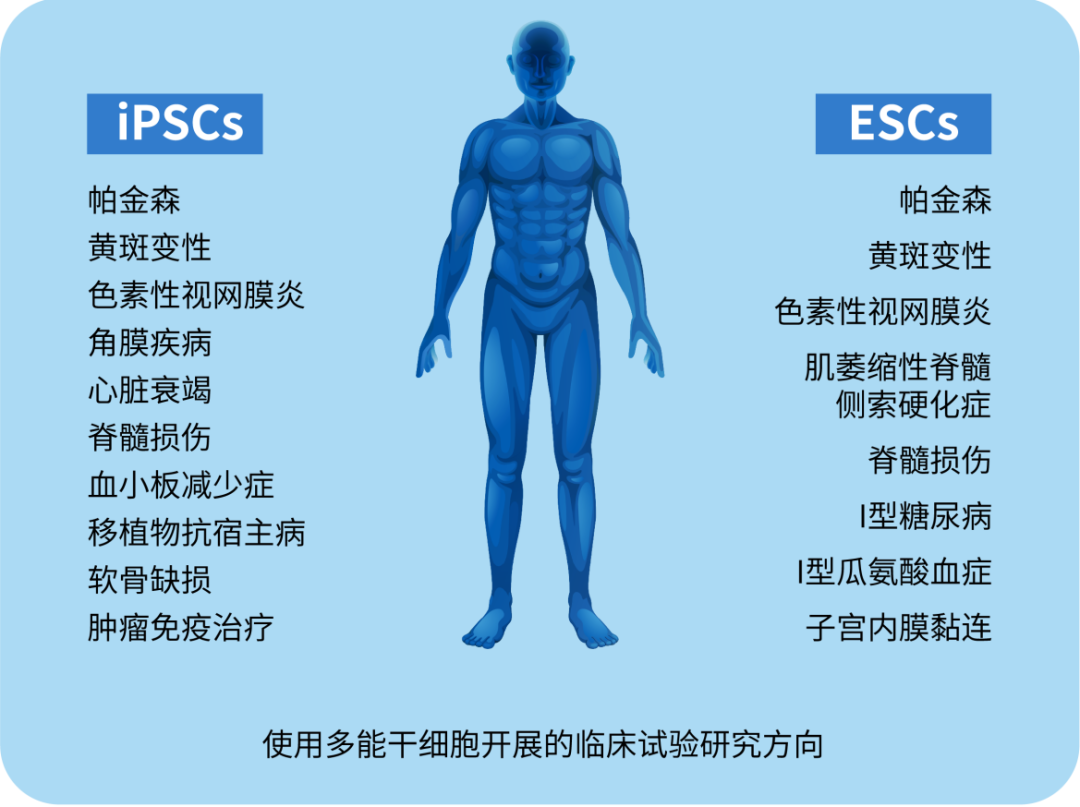
Note: Clinical trials of cell therapy using PSC
-01-History of pluripotent stem cells
Pluripotent stem cells are self-regenerative cell types that have the ability to differentiate into various cells in three germ layers. Historically, the first pluripotent cell lines were embryonic carcinoma (EC) cell lines established from human germ cell tumors and undifferentiated compartments in mice. Although EC cells are a powerful tool in vitro, these cells are not suitable for clinical application due to their cancer origin and aneuploid genotype.
In 1981, scientists created the first mouse embryonic stem cells. Mouse ESCs are derived from the inner cell mass (ICM) of preimplantation blastocyst. The blastocyst has a unique biological structure, containing the trophoblast and ICM, and eventually the ICM develops into various fetal tissues, and the trophoblast cells develop into the placenta and fetal membrane. During embryonic development, ESCs exist for only a short period of time, while in vitro, ESCs can be expanded indefinitely in an undifferentiated state. Over the past 40 years, the discovery of mouse ESCs has dramatically changed the field of biomedical research and regenerative medicine.
In 1998, Professor James Thomson of the University of Wisconsin-Madison isolated the world's first human embryonic stem cell line from blastocyst embryos, causing a huge controversy in the global media and ethical research committees because of opposition to the use of human embryos for research purposes. Studies using hESCs have demonstrated their therapeutic potential in clinical Settings. About the clinical application, but hESC ethical issues and immune rejection problems highlighted.
In 2006, Professor Shinya Yamanaka of Kyoto University in Japan and his student and assistant Kazutoshi Takahashi used four transcription factors (Oct-3/4, Sox2, Klf4 and c-Myc, known as OSKM), The first mice induced pluripotent stem cells were prepared from mouse somatic cells.
In 2007, Takahashi et al. successfully produced the first human induced pluripotent stem cells with similar molecular and biological characteristics to hESCs using the same OSKM factor.
Since then, hiPSC has been extensively studied, expanding our understanding of the pathogenesis of many diseases and helping us develop new cell therapies and personalized medicine.
-02-Clinical application of pluripotent stem cells
Since the first human embryonic stem cell line was created in 1998, research into pluripotent stem cells has made significant advances in regenerative medicine, disease modeling, drug screening, and stem cell therapy. The use of hESCs in clinical trials remains constrained by ethical concerns and strict regulation, with limited preclinical data supporting its therapeutic potential. There is no denying that hESC has achieved some success in the treatment of diseases.
In 2012, Steven Schwartz's research team reported the first clinical evidence of the use of HESC-derived retinal pigment epithelium (RPE) for the treatment of Stargardt macular dystrophy, a dry age-related macular degeneration in patients. When the RPE differentiation rate was greater than 99%, 5×10*4 RPE were injected into the subretinal cavity of each patient. Because the hESC source of differentiated RPE is exposed to mouse embryonic stem cells, it is considered a xenograft product and requires a lower dose of immunosuppressive treatment. This study showed that hESCs improved patients' vision by differentiating into functional RPE without any serious adverse events.
Subsequently, two Phase I/II clinical trials were conducted and the results were published in 2015. In the clinical trial, patients were divided into three groups and received three different doses of hESC-RPE with 10×10*4, 15× 10*4 and 50×10*4 RPE cells in one eye. After 22 months of follow-up, 19 patients had improved visual acuity, 7 patients had no improvement, and 1 patient had further decreased visual acuity. The technical challenge was the unbalanced proliferation of RPE after administration, which was observed in 72% of patients after treatment.
A similar approach was also performed in two Korean patients with age-related macular degeneration and two patients with Stargardt macular dystrophy. Study results: The safety of hESC-RPE cells was confirmed, and visual acuity improved in 3 patients.
Recently, Chinese researchers have also developed clinical-grade hESC-RPE cells in non-differentiated culture for the treatment of patients with wet age-related degeneration.
Due to the prominent ethical issues and immune rejection of hESC, hiPSC-RPE cells have become a potential source of cells for the treatment of macular degeneration. Although RPE differentiation protocols have been optimized to improve the efficacy of hiPSC-RPE cells, they remain inadequate, time-consuming, and laborious.
For clinical application, a method was developed to effectively differentiate "initiated" naive hiPSCs into RPE using temporary inhibition of the FGF/MAPK signaling pathway and non-feeding layer culture conditions. Overexpression of specific transcription factors in hiPSC throughout the differentiation process is also an interesting way to generate a large number of clinically useful RPE cells. In recent studies, overexpression of three transcription factors (OTX2, PAX6, and MITF) stimulated the differentiation of hiPSC into RPE, resulting in functional RPE cells suitable for transplantation. To date, although reported data from Phase I/II clinical trials have been sufficient to support the safety of hESC-RPE cells, they are still at an immature stage.
Therefore, future studies should look at the cellular manufacturing process of RPE and the development of subretinal drug delivery pathways to further improve the efficacy of RPE manufacturing and implantation in patients' retinas. Numerous studies have shown that HESC-derived cardiomyocytes exhibit cardiac transcription factors and exhibit both cardiomyocyte phenotypes and immature electrical phenotypes. In addition, the use of hPSC derived cardiomyocytes could provide the large number of cells needed for true remyalisation and transplantation. As a result, these cells could be a promising new treatment for cardiovascular disease in humans.
In one case report, HESC-derived cardiomyocytes showed potential therapeutic efficacy in patients with severe heart failure without any subsequent complications. This is a Phase 1 trial evaluating the safety of HESC-cardiac progenitor cells from HESC-cardiac progenitor cells in a fibrin gel scaffold for the treatment of 10 patients with severe heart failure (NCT02057900). The results are encouraging; Not only did HESC-cardiac progenitor cells meet clinical-grade standards, but their combination with tissue-engineered scaffolds could treat severe heart disease (the first patient reached a 7-year follow-up period in October 2021).
Currently, two clinical trials using HESC-derived cardiac progenitor cells are underway. What is interesting is that the results of the study will pave the way for approval for commercialization. The first trial was conducted by Prof. Yoshiki Sawa, a cardiac surgeon at Osaka University and one of the inventors of HEARTSHEET®, to make thin sheets of cardiomyocytes via iPSC for the treatment of heart failure. Currently, the research team is conducting a clinical trial to verify the safety and efficacy of cardiomyocyte flakes in the treatment of heart failure (jRCT2053190081). The clinical indication was ischemic heart disease and 10 patients were enrolled.
Another clinical trial used a collagen-based construct, BioVAT-HF, to contain HiPSC-cardiomyocytes. The trial was divided into two parts to evaluate cell doses: (Part A) enrolled 18 patients and (Part B) enrolled 35 patients to test a wide range of engineered human myocardial (EHM) doses. The expected results of this clinical trial will provide a "proof of concept" for the use of EHM in stimulating the revascularization of the human heart. To date, no adverse events or serious adverse events have been reported to support its safety. Unfortunately, the number of patients treated is relatively small and is not sufficient in terms of effectiveness.
In 2010, Geron conducted one of the first clinical trials of a pluripotent stem cell-based therapy, using human embryonic stem cell-derived oligodendrocyte progenitor cells (OPC1) to treat spinal cord injury. Clinical results: Safety was confirmed in 5 participants one year after administration. Magnetic resonance imaging showed improvements in spinal cord degeneration in four participants. Asterias biotheraptic (AST) continued Geron's research by conducting the SCi Star I/IIa Phase I study to evaluate the therapeutic effects of AST-OPC1 (NCT02302157). The results of the trial, published on clinicaltrials.gov, showed significant improvements in running speed, forelimb stride length, forelimb longitudinal deviation, and hindlimb stride frequency. Interestingly, data from a recently published multicentre, non-randomized, single-group, interventional Phase I trial showed no evidence of neurological decline, with patients developing enlarged tumors, further spinal cord injury, or nasopharyngeal formation after 10 years of OPC1 use. This dataset provides reliable evidence supporting the safety of OPC1 with an event-free period of up to 10 years, which reinforces the safety of SCi Star trials.
An analysis of global trends in clinical trials using HPSC-based therapies showed that 77.1% of the studies were observational (i.e., no cells were injected into the patient) and only 22.9% used HPSC-derived cells as an interventional therapy.
The number of studies using hiPSC was relatively higher than the number using hESCs, 74.8% versus 25.2%, respectively. Most observational studies were conducted in European and American countries, including the United States (41.6%) and France (16.8%), while most interventional studies were conducted in Asian countries, including China (36.7%), Japan (13.3%) and South Korea (10%). In terms of target diseases, it's clear. The three most studied diseases are eye diseases, circulatory diseases and neurological diseases. Surprisingly, there has been little progress in the clinical use of pluripotent stem cells since hESC was first identified globally. The relatively small number of clinical trials focusing on the use of iPSCs as therapeutics may be attributed to the potential for genomic instability, immune rejection, and tumor formation of iPSCs.
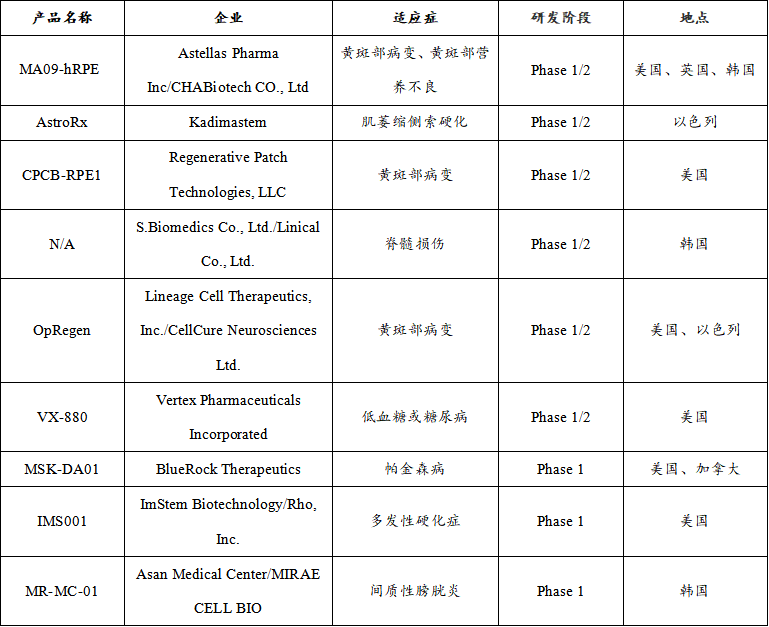
Therapeutic ESC registered clinical product
Therapeutic iPSC registered clinical products
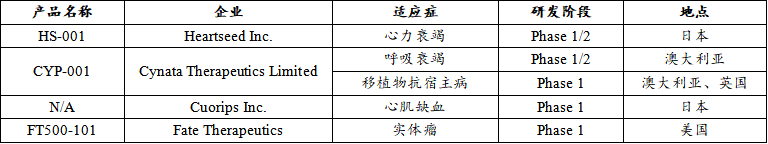
In terms of the types of diseases treated, registered clinical trials of iPSC and ESC involve a variety of indications, including type I diabetes, macular degeneration, Parkinson's disease, spinal cord injury, etc. Of the nine therapeutic ESC registered clinical products, ophthalmic diseases (33%) and neurological diseases (45%) were the main areas of study, with additional therapeutic studies in the endocrine system (11%) and urinary system (11%). Among the 4 therapeutic IPSC-registered clinical products, cardiovascular diseases (40%) accounted for a large proportion.
Summary and outlook
We provide an overview of clinical trials conducted in Japan using IPSC-based methods. While iPSC still faces many challenges, it still has great potential for cell therapy and other applications. A large number of scientists continue to work hard in this field and continue to overcome the remaining obstacles.
In recent years, domestic companies based on iPSC treatment have also sprung up, such as Chengnuo Regenerative Medicine technology, Hode Biology, Ruijian Medicine, etc., have applied to carry out IND or IIT, and began various attempts to explore China's iPSC treatment. In the near future, IPSC-based cell therapy technology will become a true clinical option, bringing benefits to patients worldwide.