
Source: Stem cell base
As of December 31, 2023, 106 clinical trial applications for stem cell drugs from 56 domestic enterprises (excluding subsidiaries) were accepted, of which 2 were added in December. A total of 79 of 44 companies (excluding subsidiaries) were granted tacit access to clinical trials (implied permission for clinical trials), of which 4 were added in December. Of the 106 accepted stem cell drugs, 15 have been unable to find IND review information or review suspended (2 new in December), and 14 are under review. In 2023, a total of 47 applications for clinical trials of stem cell drugs were accepted, and 35 were approved to enter clinical trials (clinical trial implied approval); In 2022, a total of 29 applications for clinical trials of stem cell drugs were accepted, and 26 were granted tacit approval to enter clinical trials (implied permission for clinical trials).
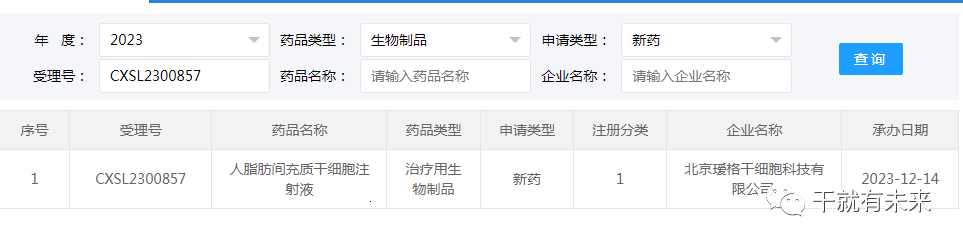
New Acceptance 1:On December 14, 2023, the clinical trial application of "Human adipose mesenchymal stem cell Injection" of Beijing AIGE Stem Cell Technology Co., Ltd. was accepted (acceptance number: CXSL2300857). This is the third indication to be admissible for this cell injection, having previously received implied approval for hand dysfunction (contracture) due to skin fibrosis of the hand in systemic sclerosis (ADmissible number: CXSL2300204) and skin sclerosis of the head, face and limbs in focal sclerma (admissible number: CXSL2300630).
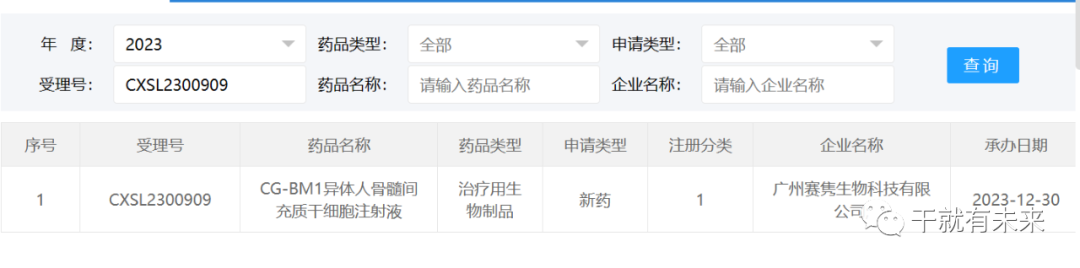
New Acceptance 2:On December 30, 2023, the application for clinical trial of "CG-BM1 allogeneic human bone marrow mesenchymal stem cell injection" of Guangzhou Saijun Biotechnology Co., Ltd. was accepted (acceptance number: CXSL2300909). This is the third indication accepted for this cell injection, which has previously received implied approval for infection-induced moderate-to-severe acute respiratory distress syndrome (ARDS) in adults (Acceptance number: CXSL2101334) and chronic acute liver failure (ACLF) (acceptance number: CXSL2200505).
Added implied license 1:Ruijian Medical Technology (Suzhou) Co., LTD. Wuhan Ruijian Pharmaceutical Technology Co., LTD. "Human Dopaminergic precursor cell injection" (IPSC-derived drug) (acceptance number: CXSL2300628) obtained the implied approval of new drug clinical trial, indication: the onset of early onset Parkinson's disease earlier than 50 years old. Previously, this stem cell preparation has been implicitly approved for clinical trials in one indication: Parkinson's disease (CXSL2100023).

Added implied license 2:"Human adipose mesenchymal stem cell Injection" (acceptance number: CXSL2300630) of Beijing AIGE Stem Cell Technology Co., LTD., obtained the implied approval of new drug clinical trial, indication: focal scleroderma head, face and limbs skin sclerosis. This stem cell formulation has previously been implicitly licensed for hand dysfunction (contracture) caused by skin fibrosis of the hand in systemic sclerosis (CXSL2300204).

Added implied license 3:Guizhou Zhongguan Biotechnology Co., LTD. 's "Human umbilical cord Mesenchymal stem cell Injection" (acceptance number: CXSL2300664) obtained the implied approval of new drug clinical trial, indication: early (ARCO stage I or II) non-traumatic necrosis of the femoral head. This stem cell formulation has previously been implicitly licensed for knee osteoarthritis (CXSL2200145).

Added implied license 4:Hangzhou Yiwensai Biotechnology Co., LTD. "Human umbilical cord mesenchymal stem cell injection" (acceptance number: CXSL2300670) obtained the implied approval of new drug clinical trial, indication: moderate and severe atopic dermatitis. This stem cell formulation has previously been implicitly licensed for moderate to severe acute respiratory distress syndrome (CXSL2300221).
