
Source: National Stem Cell Resource Bank
The National Stem Cell Resource Bank (hereinafter referred to as: Stem cell Bank), approved by the Ministry of Science and Technology and the Ministry of Finance in 2019, is a national science and technology resource sharing service platform, and belongs to the National science and technology innovation base of basic support and condition guarantee. At present, the stem cell bank has built the first standardized resource bank recognized by ISO 20387 in China, and established the ISO 17025 quality management system recognized by CNAS, which can provide standardized cell science and technology resource sharing.
The stem cell Bank established the first clinical human embryonic stem cells in China in compliance with the regulations, stored cell resources of multiple species, and established an international and standardized cell resource bank in accordance with ISO 20387 based on the international standards and norms of biobank and the framework of shared service management system. A perfect cell resource sharing platform has been built, which can provide a variety of cell sharing services. It has signed cooperation agreements on stem cell resources and technology sharing with a number of scientific research institutions, hospitals, enterprises, etc., which has strongly supported a number of major basic research and more than ten stem cell clinical studies filed by the State Two Committees and the State Drug Administration and five phase I/II clinical trials approved by the State Drug Administration. In order to meet the new demand for cell resources of users in the field, the cell resources of the stem cell bank are gradually enriched, and the cell list is updated (Table 1).
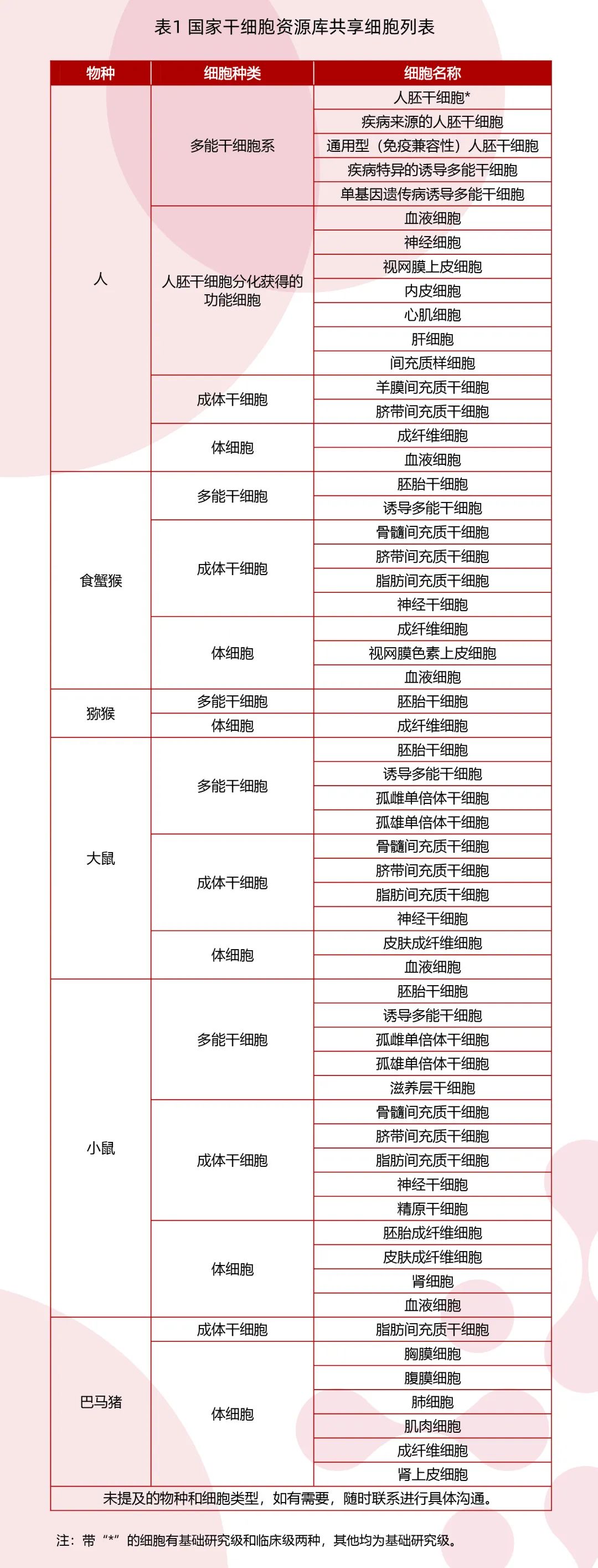
At the same time, the stem cell bank is based on the ISO 17025 quality management system, which can provide a number of quality testing sharing on cell line construction and library construction, and 16 detection methods have been approved by ISO 17025, and related testing activities have supported the construction of special projects in many countries. It has effectively guaranteed the stem cell clinical research projects for many indications such as Parkinson's disease, macular degeneration, retinitis pigmentosa, and blood diseases.