
Source: stem cells and exosomes
The advantage of iPS cells in stem cell therapy is that autologous iPS cells can be used, thus avoiding allogeneic immune rejection. However, the establishment of iPS cell lines is extremely time-consuming and costly, especially to establish clinical-grade iPS cell lines that are guaranteed by Good manufacturing practices (GMP).
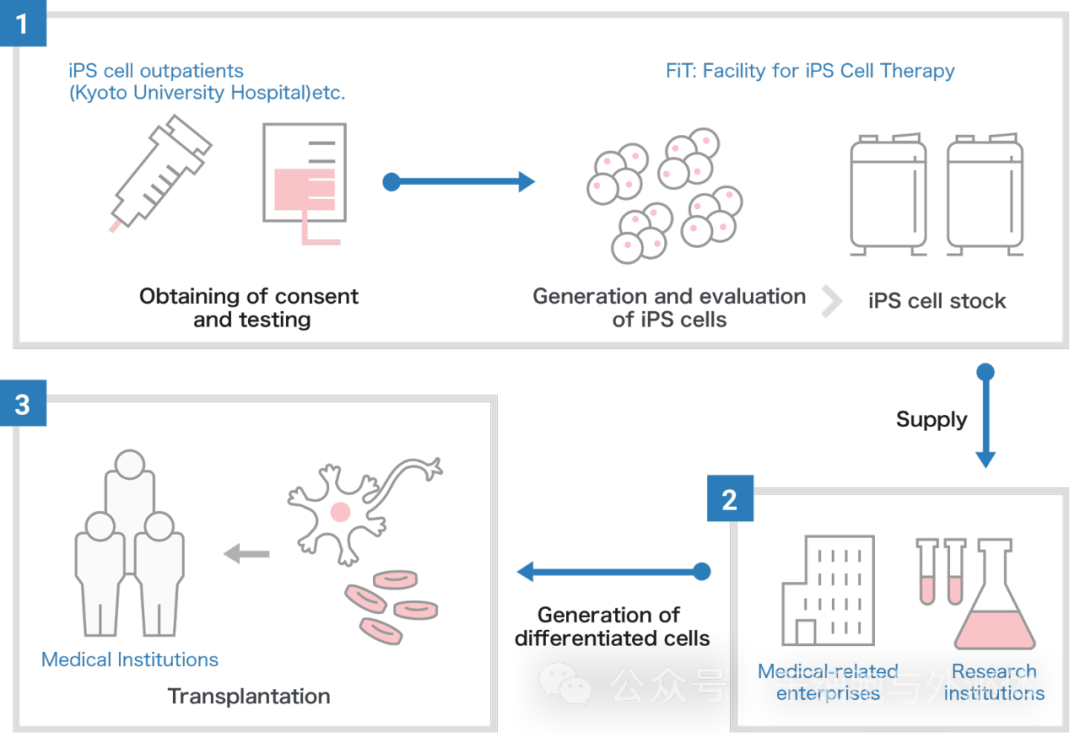
In addition, success in obtaining iPS cell lines is affected by individual differences in the donor, including age. As a result, the implementation of the Regenerative Medicine Research Center Network plans to shift to the use of allogeneic iPS cell lines derived from custom autologous iPS cells.
The Japanese iPS Cell Inventory project aims to generate a range of iPS cell lines from homozygous human leukocyte antigen (HLA) haplotypes. In terms of HLA-A, HLA-B, and HLA-DR, these homozygous cells are more widely compatible than heterozygous cells, and 140 homologous haplotype lines will cover 90% of the Japanese population.
Current situation and future direction of clinical application of iPS cells
Hiraku Tsujimoto and Kenji Osafune, researchers at The Graduate School of Medicine at Kyoto University and the International Institute for Biological Resources Research at Meiji University in Japan, published a paper titled Current status and future in the trade Journal The FEBS Journal directions of clinical applications using iPS cells - focus on Japan - Directions of Clinical Applications using iPS Cells - Focus on Japan
The report concludes that in recent years, significant advances have been made in regenerative medicine using iPS cell technology, including these technologies and their clinical applications; Advances in the establishment of iPS cells, including the HLA-homo iPS cell reserve project in Japan and the development of low-antigen iPS cells using genome editing technology, are discussed. iPS cell-based therapies in or near clinical application are described, including the treatment of ophthalmology, neurology, heart, hematology, cartilage, and metabolic diseases. The main contents of the report are as follows:
iPS cells revolutionize new technology in medicine
By discovering induced pluripotent stem (iPS) cells, Yamanaka and colleagues overcame the major ethical concerns of destroying human blastocysts associated with the use of embryonic stem (ES) cells. The study found that the combination of four factors - OCT4, SOX2, KLF4 and c-MYC (OSKM) - was sufficient to reprogram mouse and human somatic cells into iPS cells.
Because iPS cells have the potential to expand indefinitely in vitro and differentiate into any cell type in the body, they can be used to generate a wide range of differentiated cells for cell therapy, disease modeling, drug discovery, and toxicological screening.
For cell therapy, since the technique initially uses genome-integrating viruses as vectors, there is a risk that the viruses will integrate into chromosomes in locations near cancer-related genes, leading to tumorigenesis. However, safe methods have since been developed, such as using free plasmids that cannot be integrated into chromosomes, to accelerate iPS cells towards clinical use.
Thanks to recent advances in genome editing technology, disease-specific iPS cells with a genetic predisposition to cause disease can now be generated quickly and accurately. Disease modeling studies have subsequently been conducted for many difficult conditions, in which disease-specific iPS cells differentiate into damaged cell types or tissues to reproduce the disease phenotype in vitro.
iPS cells are a new technology that has revolutionized medicine. Clinical trials based on iPS cell technology are being conducted in the United States, China, Germany, and other countries (e.g., NCT03763136, NCT03759405, NCT04396899, NCT04106167, NCT03841110, and NCT04245722). More clinical trials are conducted in Japan than anywhere else. Therefore, the researchers will focus on clinical studies in Japan.
Safety clinical application technology
Establishment of iPS cells
Methods for building iPS cells can be roughly classified according to reprogramming methods, including retroviruses, lentiviruses, Sendai viruses, adenoviruses, plasmids, add-on plasmids, transposons, small ring DNA, RNA, proteins, and small molecular compounds. It can also be further classified according to efficiency and safety; The efficiency of reprogramming, the technical difficulty, and the risk of integration into the genome. In general, efficiency and safety are inversely correlated. Therefore, the preferred reprogramming method will depend on the purpose of the study. With fewer safety concerns, efficient methods are preferred for basic research. In clinical applications, on the other hand, safety is Paramount.
Development of iPS cell maintenance medium
Traditionally, the culture of human pluripotent stem cells (iPS/ES cells) has been performed in a medium supplemented with serum substitutes and fibroblast growth factor 2 (FGF2), using mouse fibroblasts as feeder cells. As a first step in the clinical application of iPS cells, a number of farm-free cell culture methods have been developed using dedicated media and extracellular matrix that does not contain farm-cells. The Center for iPS Cell Research and Application (CiRA) at Kyoto University has developed "StemFit," an iPS/ES cell culture medium that does not contain components of animal origin.
iPS cell inventory project
Kyoto University's CiRA Foundation's iPS Cell Inventory project is being developed for regenerative medicine and uses blood samples from healthy donors whose human leukocyte antigens (HLEucocyte antigens; HLA-A, HLA-B and HLA-A, HLA-B and HLA-DRB1), which makes the transplant less likely to cause immune rejection.
In the case of autologous transplantation, iPS cells are generated from the patient's blood or other somatic cells and checked for quality. Next, the iPS cells differentiate into cells for patient treatment and are transplanted. Although the iPS cell lines used in the iPS Cell Inventory project are allogeneic, they save time and cost compared to autologous transplants.
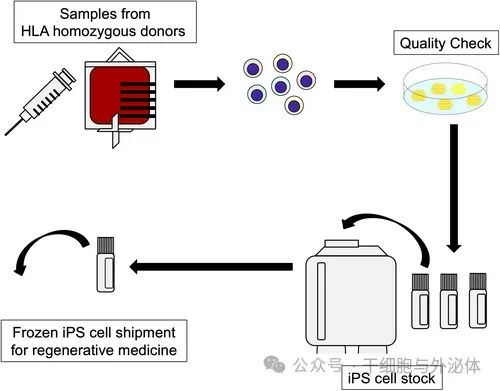
Figure: Schematic diagram of iPS cell inventory project. HLA homozygous donors donate peripheral blood from which iPS cells are generated.
In this project, the CiRA Foundation generates iPS cells in its preparation facility. Since then, it has only stored quality-assured iPS cells that have been confirmed to be safe and made them available to medical and research institutions in Japan and abroad for medical research purposes, including clinical applications.
As of March 2021, iPS cells from seven HLA homozygous (HLA-HOMO) donors are available, estimated to cover about 40% of the Japanese population. However, HLA-A, HLA-B, and HLA-DRB1 homozygous iPS cell donors are very rare. As a result, it takes a lot of time and cost to collect the 140 species that cover about 90 percent of Japan's population. In fact, it is estimated that up to 16,000 human leukocyte antigen types would have to be examined to find 140 types.
Cell therapy using iPS cells
Age-related macular degeneration and retinitis pigmentosa
Age-related macular degeneration (AMD) is a progressive disease affecting the macular area of the retina, resulting in progressive loss of central vision. Advanced AMD causes loss of central vision, leading to severe and permanent visual impairment, which can have a significant impact on quality of life and functional independence. If the disease is detected early, treatment with anti-vascular endothelial growth factor (VEGF) can stop the progression to some extent and minimize the damage; However, AMD remains the third leading cause of severe irreversible vision loss worldwide.
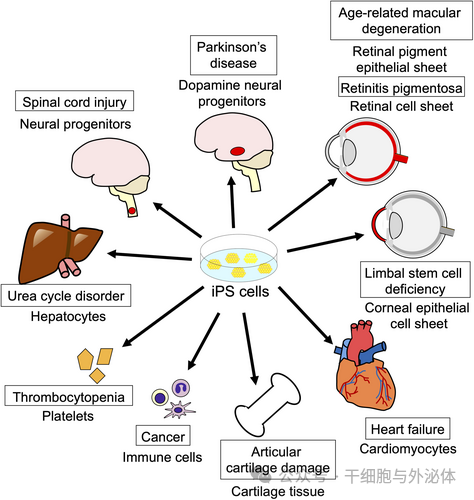
Graphic: Clinical trials of iPS/ES cell-based cell therapies are underway in Japan. The name in the box indicates the disease, and the name below the box indicates the type of iPS cell product being transplanted.
Table 1. Clinical trials currently underway in Japan. AMD, age-related macular degeneration; ALS, amyotrophic lateral sclerosis; AD, Alzheimer's disease; FOP, fibrodysplasia ossificans progressiva.
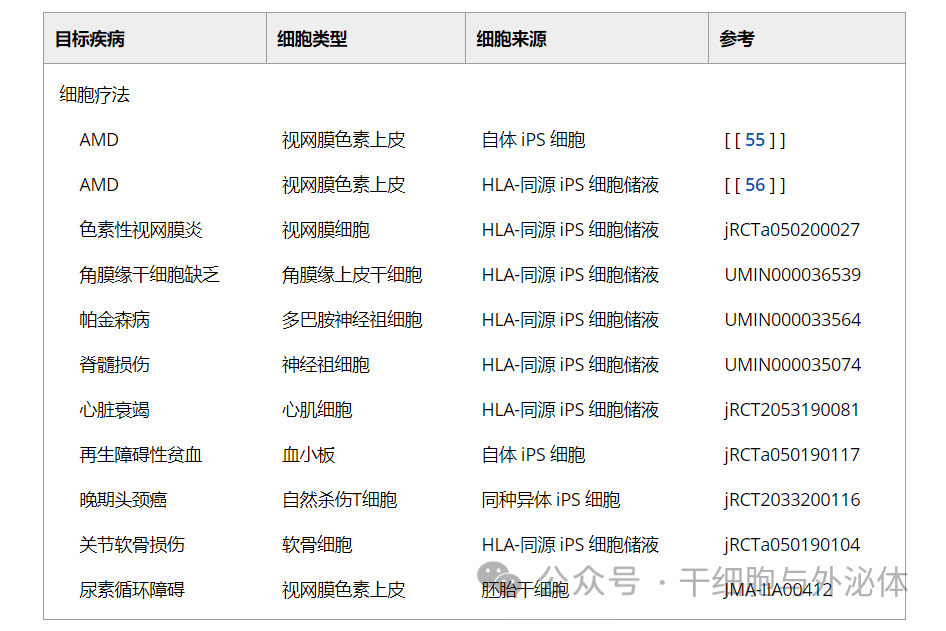
Limbal stem cell deficiency
The normal function of corneoscleral marginal epithelial stem cells is the prerequisite for the maintenance and regeneration of corneal epithelium. It is generally believed that these epithelial stem cells exist in the basal epithelium of the corneal limbus, which is the transition area between the clear cornea and the opaque sclera. For this reason, these cells are called limbal epithelial stem cells (LSCS).
The normal limbus and LSC act as a barrier to prevent the conjunctival epithelium from invading the corneal surface. LSC maintains normal homeostasis of corneal epithelium and wound healing.
When the conjunctival epithelium invades the cornea and the surface of the cornea is covered by conjunctival tissue, including blood vessels, severe corneal opacity occurs, leading to visual impairment and even blindness. LSC defects may be caused by genetic defects or secondary damage to the LSC and its niche, which may lead to abnormal corneal epithelial wound healing and/or conjunctivization of the corneal surface. Causes include burns, alkaline or acidic corrosion, Stevens-Johnson syndrome, and ocular pemphigoid. Donor corneal transplantation has been used to treat severe corneal epithelial diseases that can lead to blindness, but there are problems with rejection and donor shortages.
Parkinsonism
Parkinson's disease occurs when dopamine neurons in the midbrain substantia nigra are damaged for some reason and produce less dopamine. Parkinson's disease is the second most common neurodegenerative disorder after Alzheimer's disease (AD), with a residual lifetime risk of 2% and 1.3%, respectively, for men and women aged 40 in the United States.
Jun Takahashi's team in Japan launched a clinical trial (UMIN000033564) using iPS cell-derived dopamine neuroprogenitor cells to treat Parkinson's disease in August 2018. The cells used for transplantation were differentiated from a reserve of HLA-homo iPS cells and purified by FACS sorting CORIN, which they demonstrated is a cell surface marker that is specific for dopamine neural progenitor cells.
Prior to clinical trials, they confirmed the efficacy and safety of transplanted human iPS cell-derived dopamine neural progenitor cells in a crab-eating monkey model of Parkinson's disease. The transplanted monkeys showed increased motor capacity and improved Parkinson's symptoms. Positron emission tomography analysis revealed that the transplanted cells synthesized dopamine in the brain. In addition, no tumor formation was observed after two years of follow-up, and histological analysis of brain slides did not reveal any evidence of malignant change.
Spinal cord injury
Spinal cord injury is caused by injury or an accident that damages the spinal cord, causing the brain to be unable to transmit commands to the nerves that move the body, resulting in quadriplegia. A 2016 global survey put the number of cases of spinal cord injury at more than 20 million. In that year alone, there were about 1 million new cases worldwide, with more than 36,000 new cases in Japan. In many cases, patients are confined to wheelchairs. Currently, no treatment can fully repair the damaged area.
In 2019, Hideyuki Okano's team initiated a clinical study to transplant neural progenitor cells derived from HLA-homo iPS cell reserves into patients with subacute (1-2 weeks after injury) spinal cord injury. The main goal is to confirm the safety and efficacy of the transplanted cells. Neural progenitor cells were generated and cryopreserved. Frozen neural progenitor cells were thawed for final preparation and injected directly into the spinal cord injury area (UMIN000035074). The transplant is expected to regenerate injured nerves and restore motor function and sensation. Patients were followed up for about one year after transplantation. Since no human leukocyte antigen matching was performed, immunosuppressive drugs were given in the first six months after transplantation. Patients are also involved in rehabilitation.
Heart failure
Ischemic heart disease is the main cause of heart failure. Its pathogenesis is chronic ischemia of myocardial tissue due to coronary artery occlusion, resulting in necrosis of functional cardiomyocytes. Since mature cardiomyocytes are unable to self-renew, ischemic and necrotic areas are replaced by fibrous tissue. As a result, local wall movement was impaired and myocardial contractility decreased. To compensate for this reduction, the heart dilates, causing pressure damage to the heart muscle not directly affected by ischemia, leading to further fibrosis. This phenomenon is called remodeling and further worsens heart function.
Therefore, replenishing functional cardiomyocytes is a very sensible way to reduce the progression of heart failure. Because cardiomyocytes do not have the ability to proliferate and are difficult to produce in large numbers at once, cells similar to cardiomyocytes were used in the initial regeneration studies. Specifically, skeletal muscle cells, bone marrow-derived monocytes, and mesenchymal stem/stromal cells (MSCS) were considered candidate cells and were subjected to animal and clinical trials. Since skeletal muscle cells are not cardiomyocytes, they are expected to support contractile force by fusing with the host's heart muscle, but they can also induce arrhythmias. Mesenchymal stem cells and bone marrow cells did even worse.
Thrombocytopenia
Japan consumes more than 1.7 million liters of platelet preparations from blood donations each year. Platelet preparations can only be stored for four days after blood collection. In addition, the number of blood donors is decreasing due to fewer children and an aging population, but the number of elderly people in need of medical care is increasing. Therefore, not only platelet preparations, but also blood transfusion preparations may be in short supply in the future.
If there is severe thrombocytopenia due to aplastic anemia or other conditions, platelet transfusion is required. However, there may be an ineffective platelet transfusion, that is, the platelet count in the blood does not rise after the infusion. Reasons for this include transfused platelets being recognized as foreign and destroyed by the patient's own immune cells. In this case, platelets cannot be replaced by allogeneic platelet transfusions. However, if the platelets are made from the patient's own cells, a transfusion should prevent an immune response.
Koji Eto's group has successfully generated a large number of platelets from iPS cells using a biorereactor device and is conducting a clinical study of autologous infusion of iPS cell-derived platelets in patients with aplastic anemia who have had ineffective platelet transfusion (jRCTa050190117). The purpose of this patient trial was to test the safety of the administered platelet product. The study design was a single-center, open-label, uncontrolled/single-dose escalation study with a one-year follow-up after transfusion. The platelet transfusion process begins by collecting cells from the patient and generating iPS cells. Megakaryocytes are then generated from iPS cells and frozen as a master cell bank. After these master cells are thawed and megakaryocytes are grown in the culture medium, platelet production is performed. After platelets are separated, concentrated and washed, the remaining megakaryocytes are destroyed by irradiation and then transfused to the patient. The primary endpoint was safety and the secondary endpoint was efficacy (platelet increase).
Cancer immunotherapy
In recent years, cancer immunotherapy has gained attention as a treatment option following surgery, chemotherapy (anti-cancer drugs and hormone administration), and radiation therapy. Cd1d-restricted invariant natural killer T (iNKT) cells are lymphocytes that express a single T cell receptor (Vα24-Jα18 and Vβ11 chains in humans, and Vα14-Jα18 and Vβ8.2 chains in mice) and activate CD1d by recognizing the presented glycolipid antigens. An MHC Class I molecule.
Activated iNKT cells exhibit direct cytotoxic activity by producing cytotoxic molecules, such as perforin, while producing large amounts of cytokines, such as IFNγ, that induce the activation of NK cells and CD8+ T cells, thereby exerting anti-tumor effects. As a new treatment for head and neck cancer, the Haruhiko Koseki and Shin-Ichiro Fujii groups are conducting clinical trials using iPS cell-derived NKT cells (jRCT2033200116).
Articular cartilage injury
Osteoarthritis is the most common joint disease worldwide, affecting about 10 percent of men and 18 percent of women over the age of 60. Articular cartilage has a unique and smooth structure that allows us to move our joints smoothly and painlessly. Damaged articular cartilage can cause pain when moving the joint.
Noriyuki Tsumaki's group has initiated a clinical trial for the regeneration of joint cartilage injury through HLA-homo iPS cell-derived cartilage transplantation (jRCTa050190104). In this clinical study, only one knee was transplanted and four patients who met the criteria (ages 20 to 70 years with limited scope of cartilage damage) were included. The surgery used arthroscopic grafts of cartilage. The patient will recover for 6 weeks after surgery, and the cartilage regeneration and repair will be followed up for 1 year. The team has successfully used human iPS cells to produce high-quality cartilage.
Previous animal studies have confirmed that iPS cell-derived cartilage does not produce tumors and can regenerate cartilage in the transplanted area. The objective of this clinical study was to evaluate the safety of cartilage implantation in patients with cartilage injury to the damaged area of the knee joint. The primary endpoints were the frequency of adverse events and the presence of tumorigenesis. To prepare cartilage, chondrocytes are induced from a reserve of HLA-homologous iPS cells and cartilage tissue is generated from chondrocytes. The researchers aim to treat knee osteoarthritis with this therapy in the future.
In conclusion, clinical medicine using iPS cells has progressed safely and effectively. In this report, the researchers provide an analysis and summary of clinical trials using iPS cells in Japan and explain how gene-edited low-antigen iPS cells have the potential to solve many immunological problems in cell therapy. In addition, these iPS cells can be used as a source of a single batch of cellular products to treat the more widely distributed types of human leukocyte antigens in a patient's body, thereby reducing the cost per dose of treatment. As the risk of immunity evasion increases, cell tumorigenicity studies also need to be validated on a variety of cells and applications.
With the establishment of low-antigen iPS cell lines and individual patient iPS cell lines, cell therapy is expected to expand to a variety of diseases. In addition, developing methods to generate functionally mature cells or tissues from iPS cells, as well as human-sized organs, will facilitate novel regenerative therapies and more suitable disease models. In the next few years, regenerative medicine based on iPS cell technology will be further popularized.