
Source: CDE, official website of each company
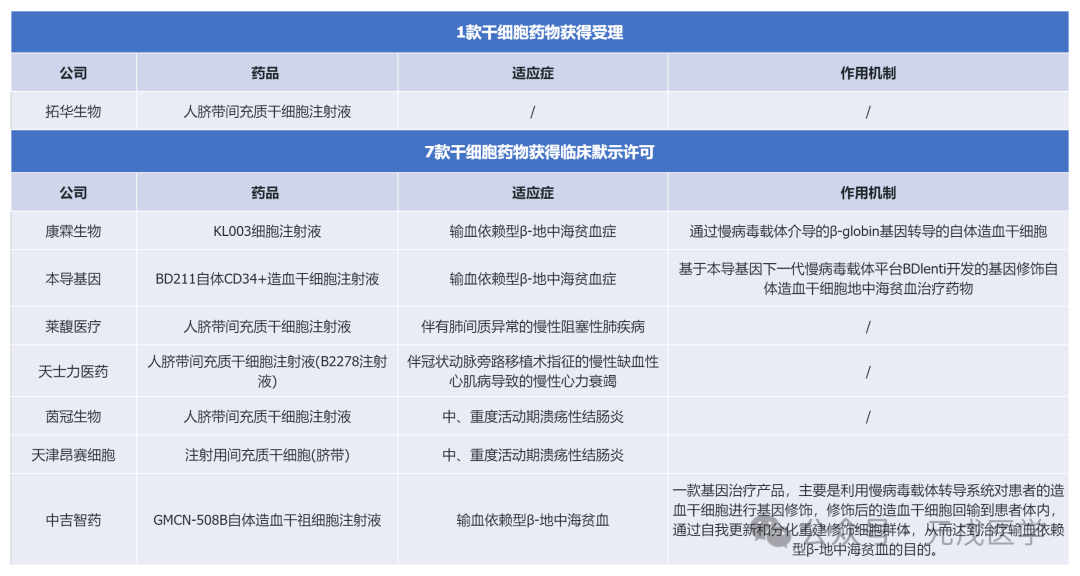
In January 2024, the Center for Drug Evaluation (CDE) of the State Food and Drug Administration accepted 1 stem-related drug, human umbilical cord mesenchymal stem cell injection; Clinically approved 7 stem cell-related drugs, including hematopoietic stem cell injection, umbilical cord mesenchymal stem cell injection, etc.
Human umbilical cord mesenchymal stem cells (hUC-MSCs) have advantages such as strong proliferation ability, low immunogenicity, convenient collection of materials, and little ethical controversy. Research results show that they have immune regulation and tissue repair functions, and show certain therapeutic potential in the treatment of lung injury diseases, and are expected to make breakthrough progress. At present, their role is attracting more and more attention.
Hematopoietic stem cells (HSCs) are a kind of adult stem cells with the ability of self-renewal and multi-differentiation to produce mature blood cells of different lineages. Autologous hematopoietic stem cell transplantation is a process in which the patient's own stem cells are collected and preserved, then pre-treated and subjected to intense chemotherapy, and then the preserved stem cells are injected back into the patient. It is mainly used to treat lymphoma, multiple myeloma and leukemia. This technology belongs to the third type of high-risk technology, which is a cellular technology and a restrictive technology.
Jilin Tuohua Biotechnology Co., Ltd.
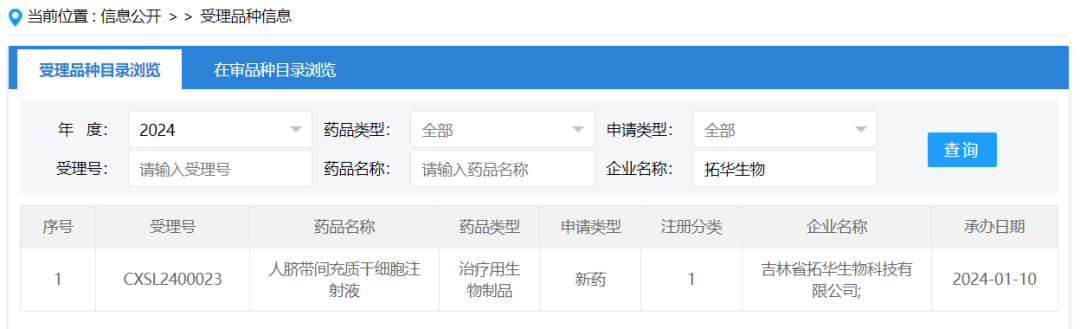
On January 10, 2024, the clinical trial application of "Human umbilical cord mesenchymal stem cell injection" submitted by Jilin Tuohua Biotechnology Co., Ltd. according to new drug Class 1 was accepted, acceptance number: CXSL2400023. This is the second indication of this cell injection to be accepted, after its wholly-owned subsidiary Suzhou Tuohua Biotechnology Co., Ltd. has obtained clinical implied approval for "human umbilical cord mesenchymal stem cell injection" for moderate/severe acute respiratory distress syndrome, acceptance number: CXSL2300152.
Jilin Tuohua Biotechnology Co., Ltd. was established in December 2010, is a professional engaged in stem cell basic research, clinical application research and stem cell technology services of biological high-tech limited company, committed to a variety of refractory diseases such as Parkinson's disease, cerebral palsy, diabetes, femoral head necrosis and tumor and other diseases of cell product research and clinical application research. In Shanghai, Shenzhen and other frontier cities invested in the construction of a number of brain disease centers, kidney disease hospitals and anti-aging centers and other medical institutions, while the development of independent brands "Vike Meilun" and "One Scission time" cell repair active peptide and superoxide dismutase protein powder series of beauty cosmetics and health food.
Kanglin Biotechnology (Hangzhou) Co., Ltd.
On January 3, 2024, Kanglin Biotechnology (Hangzhou) Co., Ltd., according to the new drug class 1 submitted "KL003 cell injection" (autologous hematopoietic stem cells via lentiviral vector mediated β-globin gene transduction) was granted the implied approval of new drug clinical trial, acceptance number: CXSL2300699, the indication is transfusion-dependent beta-thalassemia. This is the first stem cell drug implicitly licensed by Kanglin Biochem.
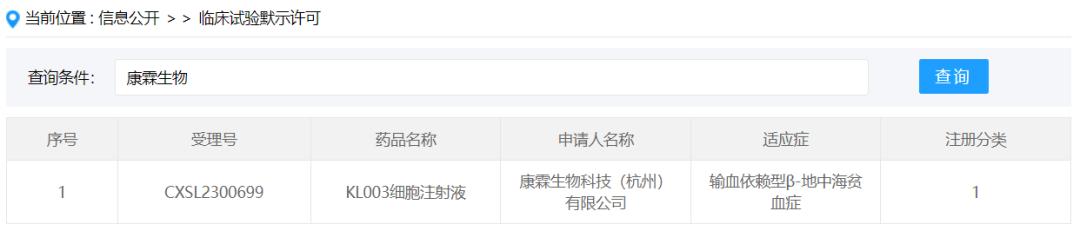
KL003 cell injection is a genetically modified autologous hematopoietic stem cell product. Autologous hematopoietic stem cells are transduced by β-globin gene mediated by lacrovirus vector and transfused back to differentiate red blood cells with normal expression of β-globin in the patient, restore the hemoglobin level of the patient, and thus get rid of the dependence on blood transfusion. Achieve a one-time functional cure effect.
Founded in August 2015, Kanglin Biotechnology (Hangzhou) Co., Ltd. is a state-level specialized new small giant enterprise aiming to make the first drug and the best innovative gene drug of the same kind. The Parkinson's disease gene therapy project led by Kanglin Biology has completed the preclinical research and is preparing to carry out the investigator-initiated clinical trial.
Shanghai Bendao Gene Technology Co., Ltd.
On January 3, 2024, Shanghai BD211 Autologous CD34+ hematopoietic stem cell injection submitted by Shanghai Bendao Gene Technology Co., Ltd. according to new drug Class 1 was granted the implied approval of new drug clinical trial, acceptance number: CXSL2300710, the indication is transfusion-dependent β-thalassemia. BD211 is the second pipeline of this gene to obtain IND approval, therefore, this gene has entered the registered clinical trial stage on the two major gene therapy routes of gene editing and gene compensation.
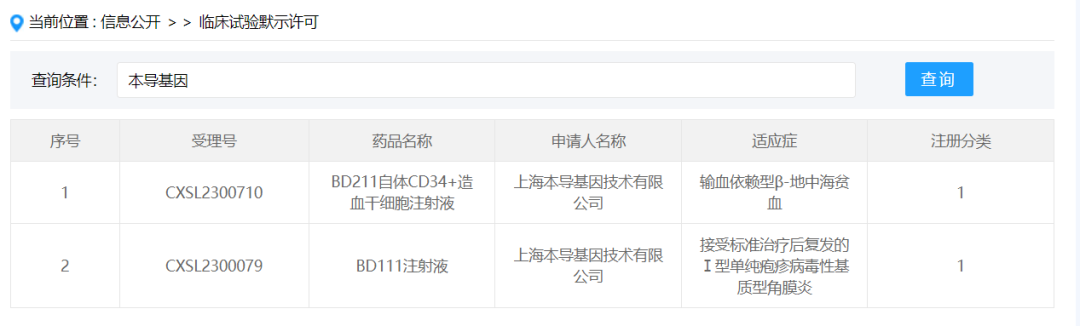
BD211 is a genetically-modified autologous hematopoietic stem cell thalassemia treatment drug developed on the basis of BDlenti, the next generation lentiviral vector platform of this gene. It has the following advantages and characteristics: Firstly, by introducing a patented insulator design, it reduces the risk of integrated mutation of the lentiviral vector and improves product safety; Secondly, the expression sequence of β-globin gene was optimized, and the hemoglobin of patients was repaired to the physiological state of healthy people through gene compensation, and the therapeutic effect was improved.
Founded in July 2018, the company is an innovative gene therapy drug research and development enterprise featuring in vivo gene editing therapy, and is committed to developing innovative drugs of global significance for refractory diseases in multiple fields such as ophthalmology, nervous system, hematopoietic system, viral infection and tumor. This gene has the world's leading VLP mRNA delivery platform (BD-VLP) and the next generation lentiviral vector platform (BDlenti). A number of First-in-Class product pipelines have been laid out around the core delivery technology platform, and a number of First-in-Human clinical studies have been carried out. Pipelines BD111 and BD211, based on two core delivery technologies, have entered the formal clinical phase.

Shanghai Laifu Medical Technology Co., Ltd.
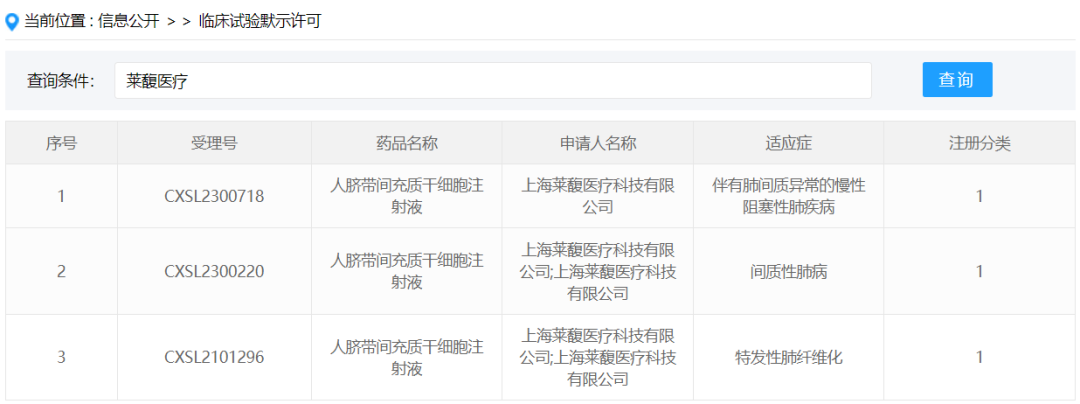
In January 2024, Shanghai Laifu Medical Technology Co., Ltd. obtained the implied approval of new drug clinical trial for "Human umbilical cord mesenchymal stem cell Injection" submitted by New drug Class 1, acceptance number: CXSL2300718, and the indication is chronic obstructive pulmonary disease with pulmonary interstitial abnormalities. This stem cell formulation has previously been implicitly licensed for idiopathic pulmonary fibrosis (acceptance number: CXSL2101296) and interstitial lung disease (acceptance number: CXSL2300220).
Founded in November 2016, Shanghai Laifu Medical Technology Co., Ltd. is a biopharmaceutical company with a full clinical stage of innovative cell and gene drug (CGT) discovery, research and development, process development and production, with a forward-looking layout of new drug development in the fields of cell therapy, gene therapy, tissue repair and regeneration.
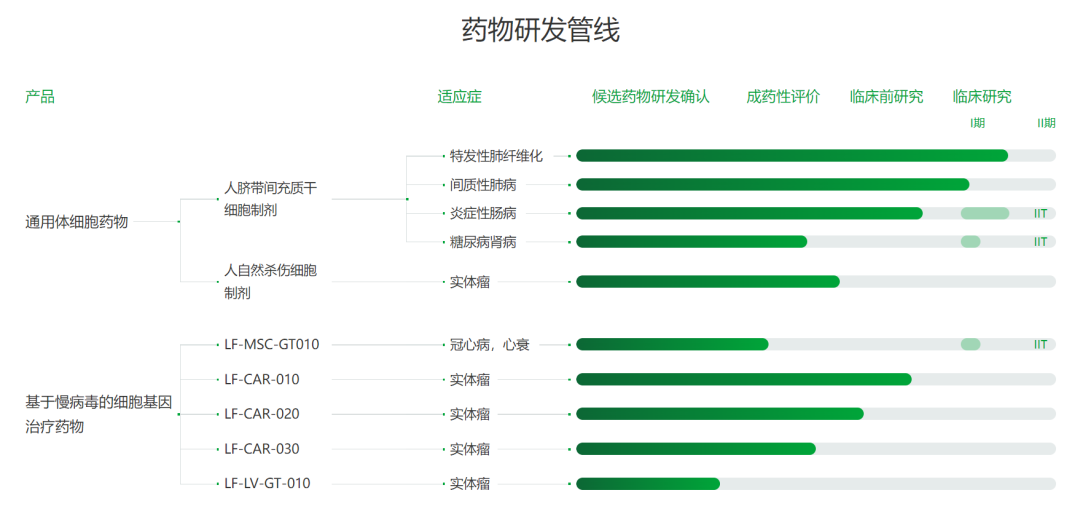
Tasly Pharmaceutical Group Co., Ltd.
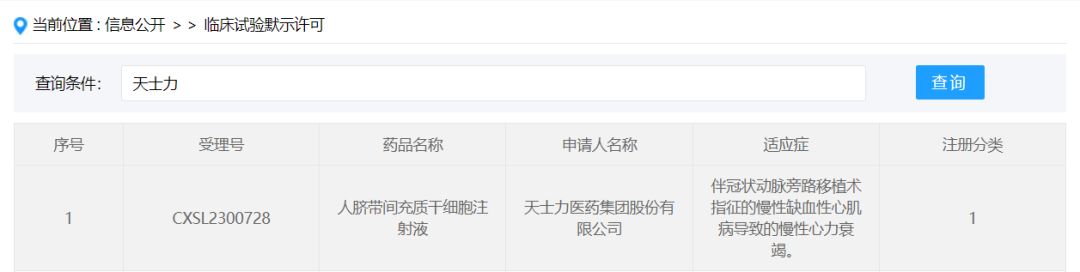
On January 18, 2024, the "Human umbilical cord mesenchymal stem cell Injection (B2278 injection)" submitted by Tasly Pharmaceutical Group Co., Ltd. under the new drug Category 1 was granted the implied approval for clinical trial of a new drug, acceptance number: CXSL2300728, indicating chronic heart failure caused by chronic ischemic cardiomyopathy with coronary artery bypass grafting. This is Tasly's first implicitly licensed stem cell drug.
2278 injection was developed by Shanghai Dongfang Hospital (Dongfang Hospital Affiliated to Tongji University). In August 2022, Tasly Pharmaceutical Group Co., Ltd. and Dongfang Hospital signed a Technology transfer (Cooperation) Contract, under which Tasly transferred the relevant technologies and achievements of B2278 injection, and gave priority to drug registration and application and follow-up clinical trial development in China on a global scale. Preclinical studies have demonstrated that B2278 injection can regulate myocardial microenvironment through paracrine effect, significantly inhibit myocardial cell tissue injury in ischemic cardiomyopathy, increase cardiac function, promote vascular regeneration, and reduce myocardial apoptosis.
Tasly Pharmaceutical Group Co., Ltd. was founded in April 1998. Its main business includes modern Chinese medicine, biological medicine, chemical medicine and stem cell and regenerative medicine. It makes use of the synergistic development advantages of modern Chinese medicine, biological medicine and chemical medicine to innovate drugs, and continues to focus on the three major therapeutic fields of cardiovascular and cerebrovascular, digestion and metabolism, and anti-tumor, which are the largest and fastest growing in the Chinese market.
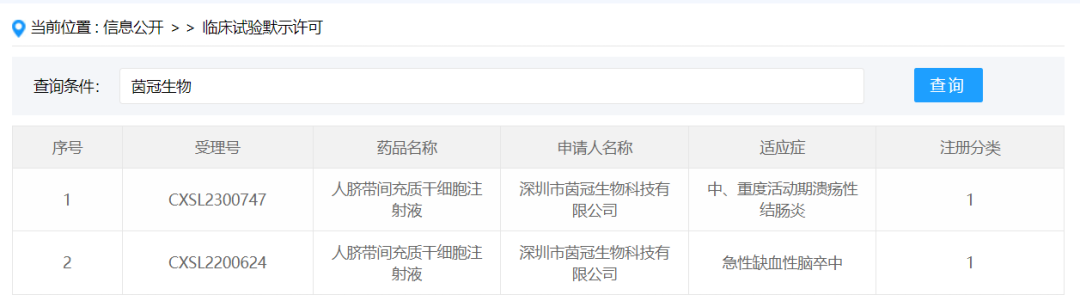
Shenzhen Yinguan Biological Technology Co., Ltd.
On January 22, 2024, Shenzhen Yinguan Biological Technology Co., Ltd.obtained the implied approval of new drug clinical trial for "Human umbilical cord mesenchymal stem cell injection" submitted by new drug Class 1, acceptance number: CXSL2300747, and the indication was moderate to severe active ulcerative colitis. Previously, this stem cell preparation has been implicitly approved for acute ischemic stroke, acceptance number: CXSL2200624.
Founded in March 2013, Shenzhen Yinguan Biological Technology Co., Ltd. is committed to cell pharmaceuticals and human health. The first phase I/IIa clinical study of "Human umbilical cord mesenchymal stem cell injection" in the treatment of acute ischemic stroke in three centers including Beijing Tiantan Hospital has successfully completed the first patient enrollment and completed the dose-limited toxicity (DLT) evaluation. At the same time, the rest of the drug pipeline is advancing.
Tianjin Angsai Cell Gene Engineering Co., Ltd.
On January 25, 2024, Tianjin Angsai Cell Gene Engineering Co., Ltd. a subsidiary of Hanlian Pharmaceutical Sector under Hanshi United, obtained the implied approval of new drug clinical trial of "mesenchymal stem cells for injection (umbilical cord)" submitted by the new drug Class 1, acceptance number: CXSL2300760, and the indication is moderate to severe active ulcerative colitis. Previously, this stem cell preparation has been implied approved for graft-versus-host disease (acceptance number: CXSL1800101), chronic acute (subacute) liver failure (acceptance number: CXSL2000335), acute respiratory distress syndrome (acceptance number: CXSL2100056), and traumatic spinal cord injury (acceptance number: CXSL2200553).
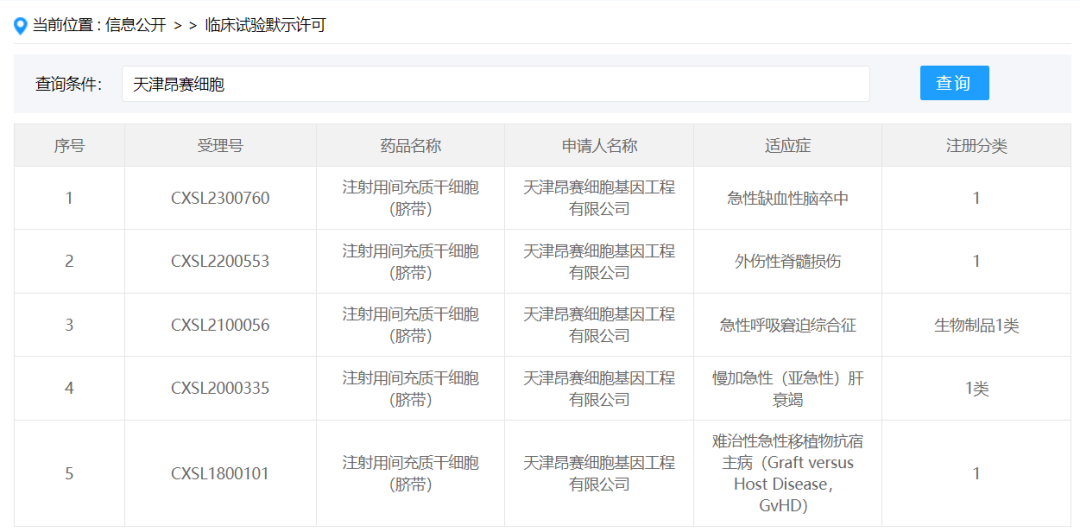
Founded in October 2004, Tianjin Angsai Cell Gene Engineering Co., Ltd. is an absolute holding subsidiary of Hanshi United Group, mainly engaged in stem cell drug research and development, tissue engineering products related to regenerative medicine and cell quality evaluation technology development. In 2006, the umbilical cord mesenchymal stem cell bank was established, with good stem cell engineering technology development capabilities, and 7 key industry technology platforms such as adult stem cell isolation and large-scale expansion of adult stem cells were established.
Zhongji Zhiyao (Beijing) Biotechnology Co., Ltd.
On January 26, 2024, the "GMCN-508B Autohematopoietic stem Progenitor cell injection" submitted by Zhongjizi Pharmaceutical (Nanjing) Biotechnology Co., Ltd. under the new drug Class 1 was granted the implicit approval of new drug clinical trial, acceptance number: CXSL2300761, for the treatment of transfusion-dependent beta-thalassaemia. This is the first stem cell drug implicitly licensed by Zhongji Zhiyao.
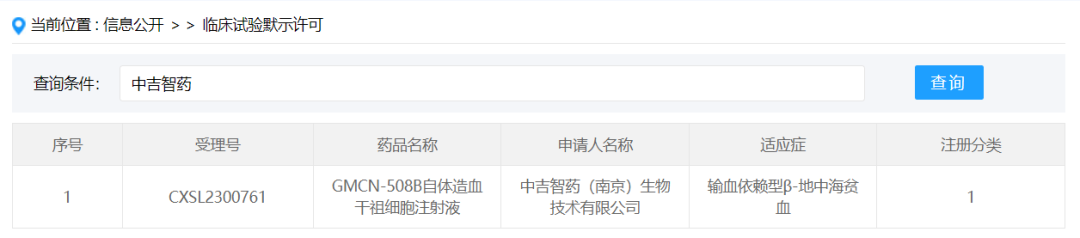
508B is a gene therapy product, which mainly uses the lentiviral vector transduction system to genetically modify the patient's hematopoietic stem cells, and the modified hematopoietic stem cells are injected back into the patient's body to modify the cell population through self-renewal and differentiation, so as to achieve the purpose of treating transfusion-dependent β-thalassemia.
Founded in September 2020, Zhongji Zhiyao (Beijing) Biotechnology Co., Ltd. is a gene therapy innovative drug enterprise focusing on hematopoietic stem cell-related diseases. The company has completed several rounds of financing and entered the clinical stage.
As more and more new stem cell drugs enter the clinical stage, we expect that stem cell therapy will be widely used in the medical field in the future.