
Source: Biotechnology Science Exchange
Many clinical studies or clinical trials have shown that mesenchymal stem cells (MSC) can treat a variety of diseases, especially some difficult diseases, but the efficacy is different. Researchers began to reflect calmly: Why the therapeutic function of mesenchymal stem cells have a greater difference in clinical efficacy? For this problem, different researchers have different thinking and interpretation.
There are many factors that affect the efficacy of mesenchymal stem cells (MSC). This article mainly discusses the key factors related to MSC: cell quality, injection route, optimal dose, and treatment timing. Other influencing factors include the type of disease the patient has, the medical skills of the doctor and the overall strength of the hospital. Mesenchymal stem cells (MSC) are hereinafter referred to as MSC.
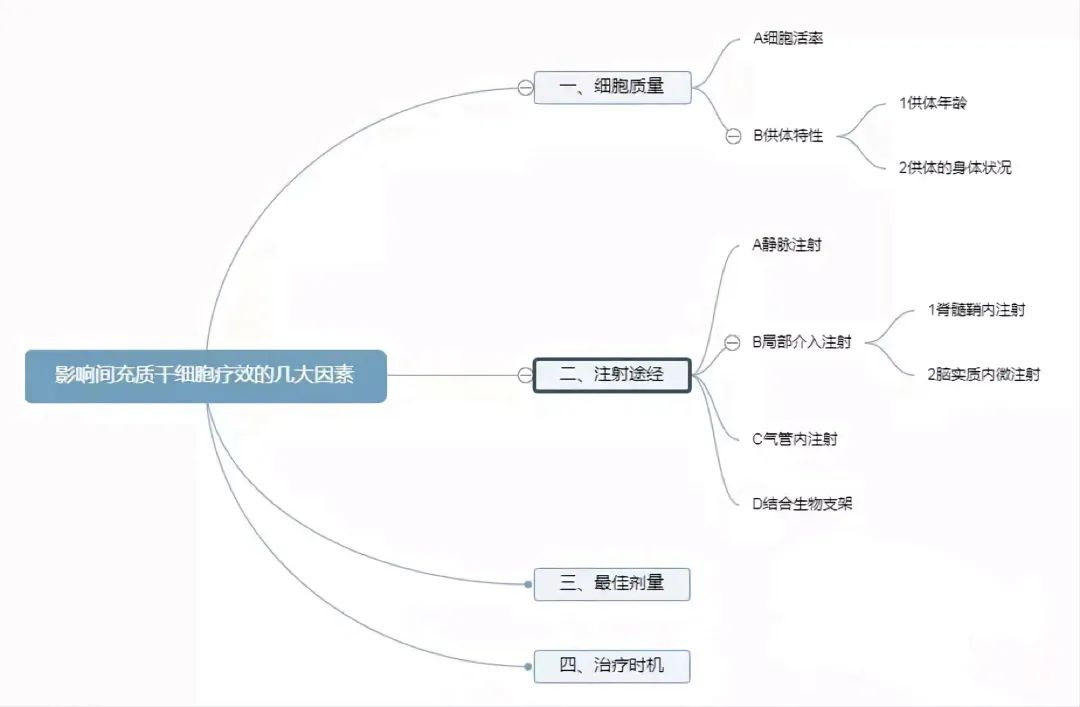
Cell mass
There are some differences in the quality of the same drug produced by different manufacturers, especially when compared with imported drugs and domestic drugs, the same situation also has different stem cell companies. To roughly define "cell mass" as the biological potency of a unit or individual cell; The higher the potency, the better the cell quality. There are several parameters that reflect the quality of mesenchymal stem cells, such as cell viability, donor characteristics, cloning-forming capacity, cell size, immunosuppressive capacity, and cytokine secretion.
1. Cell viability
How many MSCS are still alive in the MSC injection before it enters the human body?
The cell viability rate used in different clinical studies is different, the cell viability rate is >80%, >85%, 88.2± 6.1%, 90%-97%, >92%, >95%, and there are >70% cases. Cellular preparations with a survival rate of more than 90% and cellular preparations with a survival rate of only 70%, in the case of the same disease, the therapeutic effect will inevitably have a large difference. MSCS need to be alive and clear the lungs in order to work well.
2. Donor characteristics
Donor characteristics include "donor age" and "donor's physical condition."
(1) Donor age
Donor age is an important factor, as MSCs from younger donors appear to have greater viability, proliferation potential, and antioxidant capacity, while older adult-derived MSCS have lower proliferation capacity.
In terms of age, the umbilical cord, cord blood and placenta should be the most advantageous, the pulp of milk teeth is second, and the bone marrow and fat are relative to age.
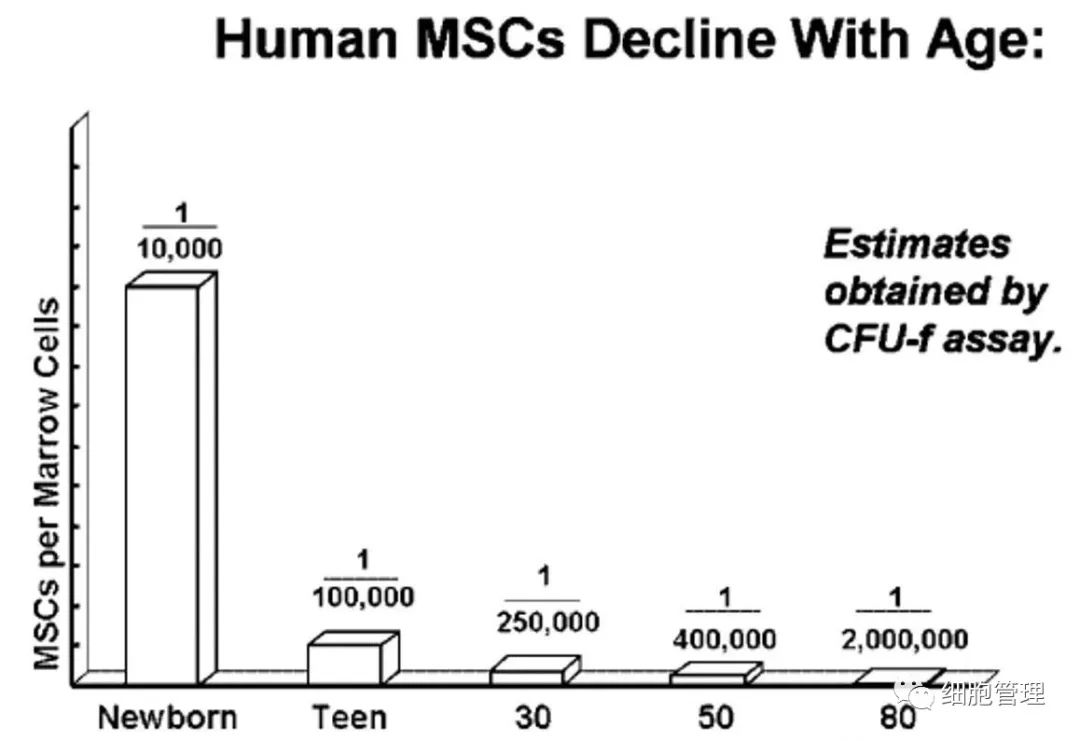
The older you get, the fewer stem cells you have in your bone marrow: at birth, one in 10,000 mononuclear cells in your bone marrow is an MSC; By age 30, the number of MSCS was reduced to 1 in 250,000 mononuclear cells in the bone marrow. By the age of 80, the number of MSCS is even smaller, with only 1 MSC in 2 million mononuclear cells in the bone marrow.
Sex may have an effect on some functions of MSC.
For example, studies have shown that female-derived MSCS express higher levels of IFN-γR1 and IL-6β, and thus have stronger immunosuppressive ability.
(2) The body condition of the donor Many studies have proved that diseases can also affect the function of autologous mesenchymal stem cells, especially in some patients with autoimmune diseases, the function of their own bone marrow MSC is abnormal, including slowing down the proliferation rate, reducing the ability to clone formation, decreasing the ability to suppress immunosuppression, reducing the amount of secreted growth factors and other pathological changes. Making the patient's own bone marrow MSC unsuitable for the treatment of their own disease.
In theory, it is possible that there is some congenital genetic variation that causes a defect in the function of the MSC, so that the MSC from the umbilical cord, cord blood and placenta at birth is not suitable for autologous treatment. No such articles have been reported, but it is a theoretical possibility.
Injection route
1. intravenous injection (systemic input)
Intravenous injection is the most commonly used and easiest route, and allows for the importation of large amounts of MSC. Note that when patients use MSC, it is best to increase the partial pressure of blood oxygen in the lungs, at least in an environment with normal oxygen content.
2. Local interventional injection
Local interventional injections, including the use of combined biomaterials (such as bone scaffolds for orthopedic disorders), intramedal injections for neurological diseases, and intratracheal injections for respiratory diseases, all favor the avoidance of pulmonary clearance of MSC.
(1) Intrathecal injection Another common input route for MSC use is intrathecal injection. Intrathecal injection of MSC is commonly used to treat neuropathic disorders, including stroke, cerebral palsy, autism, etc., and this technique can be used in most children, including premature infants.
It was reported that umbilical cord derived MSC was intramural injected into 8 pairs of twins with cerebral palsy. All patients received 4 intramural injections 3-5 days apart, each (1.0-1.5) ×107 MSC, and motor function improved significantly after 6 months of treatment. Another clinical study has shown that intravenous and/or intrathecal injection of allogeneic MSC can improve muscle tone, strength, language, memory, and cognitive abilities in children with cerebral palsy. When MSC is injected intrathecally under general anesthesia, there will be adverse reactions related to the infusion, fever and vomiting are the most common, and even more severe seizures. However, all symptoms resolved spontaneously within 72 hours, and no further complications occurred during the 6-month follow-up period. It has been speculated that fever and vomiting may be related to general anesthesia.
(2) Intracerebral parenchymal microinjection In the clinical study of MSC in the treatment of cerebral palsy, researchers evaluated the feasibility and effectiveness of intravaginal injection combined with intracerebral parenchymal microinjection of MSC in the treatment of cerebral palsy. In this clinical study, autologous bone marrow MSC were cultured in vitro to 4-5 generations, using a dose of 2X107 MSC per injection; All patients received intravaginal MSC, but patients with older age or larger head (5 years old or with a head circumference of 50 cm or larger) first received two intravaginal injections and then underwent stereotactic surgery to receive intracerebral MSC microinjection therapy. Total motor function scores were improved in all patients to varying degrees, but intraparenchymal microinjection did not bring additional benefits. The researchers observed only transient hypothermia and wound pain, but no more serious adverse events.
A clinical study of local injection of bone marrow MSC around cerebral ischemic area for the treatment of stroke (more than 6 months of onset) included 18 patients, all of whom did not receive rehabilitation treatment. After 1 year of observation and evaluation (ESS, NIHSS, mRS And F-M total scores and motor function scores), various scores were improved. However, all patients experienced varying degrees of side effects from local injection (which were analytically independent of MSC), including headache, nausea, vomiting, depression, increased muscle tone, fatigue, elevated blood sugar, and elevated C-reactive protein.
(3) Intratracheal injection of preterm infants is often associated with the risk of bronchopulmonary dysplasia (BPD). A small clinical trial demonstrated the feasibility of MSC intervention in premature infants with BPD. The average weight of the nine preterm infants, who averaged 25.3 weeks gestation, was 793 grams; The MSC dose of the first 3 BPD children was 1X107/kg, and the MSC dose of the last 6 BPD children was 2X107/kg. After 7 days of treatment, the concentration of inflammatory factors in bronchial secretions decreased significantly, and the Respiratory Severity Score improved significantly. (4) Combining biological scaffolds with stem cells to treat refractory diseases, especially nerve injury diseases, is a new therapeutic means.
Nanjing Gulou Hospital carried out a clinical study, MSC combined with collagen stent for intrauterine transplantation, treatment of uterine adhesion, 30 months later, 26 patients of 10 successful pregnancy, 8 pregnant women successfully gave birth to the baby, 1 pregnant woman for 3 months, 1 pregnant woman had a spontaneous abortion.
Optimum dose
The optimal dose of MSC depends on the different disease and severity as well as the route of importation. In the clinical research and application of MSC, cell dose may be one of the most absurd and least scientific links, even if there are some clinical studies involving dose climbing experiments, but it is not based on animal experiments. Due to the huge difference in the characteristics of MSC and traditional drugs, there are at least two specific manifestations.
① After MSC enters the body, it does not conform to the typical distribution and metabolism model of traditional drugs; Traditional drugs are passively distributed, while MSC has the function of active chemotaxis to the injured site, and the distribution of MSC in healthy and diseased bodies is also different.
(2) Animal experiments of traditional drugs require multiple doses to maintain stable blood concentrations, while animal experiments of MSC are often a single injection, so that clinical studies of MSC often adopt a single injection scheme. In fact, a single injection of MSC does not achieve a good stable long-term therapeutic effect, even if there is a significant improvement in the short term.
In current clinical studies, the dose range of MSC used is very large, with the number of MSC cells used per patient ranging from 40 million to hundreds of millions of MSC.
Local interventional treatment, the lowest dose appeared in the clinical cases of MSC in the treatment of femoral head necrosis, Korea and France each clinical study dosage of more than 4500 MSC. The highest dose of interventional therapy appeared in a clinical study in China for the treatment of diabetic bullosa of the limb with 860 million cells for MSC; The second highest dose was 200 million MSCS injected into the myocardium. More than 100 million MSCS have been used for local injection in clinical studies: 120 million MSCS for Crohn's disease intestinal fistula, 100 million MSCS for articular injection for knee osteoarthritis, and 100 million MSCS for ischemic cardiomyopathy.
The cell dose injected intravenously is relatively stable, often using several million MSCS per kilogram of body weight, i.e. (1-10) x106/kg. The highest dose of intravenous infusion is 10x106/kg co-transplanted with hematopoietic stem cells, which requires 600 million MSC according to the weight of 60 kg. There is also 8x106/kg for GVHD [15,16]. The lowest dose of intravenous infusion was 0.3x105/kg in clinical trials of co-transplantation of MSC and hematopoietic stem cells. Although some experts consider 5x106/kg and 8x106/kg to be high doses, the definition of what dose is "high dose" has not yet been discussed and proven.
If MSC is regarded as a "drug", then there must be a range in which the higher the dose, the better the effect; Then, after reaching a saturated dose, continuing to increase the cell dose does not bring more efficacy, but may bring some adverse reactions.
Treatment opportunity
The timing of MSC input is also important: Should MSC be prophylactic or therapeutic?
MSC promotes the healing of diabetic feet, and the healing of ulcerated surfaces is not accompanied by scar tissue proliferation, suggesting that MSC can inhibit scar tissue proliferation, not only for the treatment of skin trauma (including plastic surgery), but also for the prevention of postoperative scar hyperplasia in pioneering procedures, such as abdominal postoperative intestinal adhesion, which is the most common. It is important to note the timing of the application of MSC, because MSC does not eliminate scar tissue, only prevent the appearance of scar tissue. Cell drugs are very different from traditional chemical drugs in that cells are alive and chemical drugs are dead. As a living cell, MSC enters the body and must interact with the microenvironment in the body.
In pathological conditions, the ischemic anoxic microenvironment is conducive to the secretion of more growth factors by MSC, and inflammatory factors (TNF-α and IL-1β) can promote the secretion of cytokines (IL-1A, RANTES, G-CSF) by MSC. However, in general, the effect of inflammatory environment on MSC is more harmful than beneficial. Because inflammatory factors can also cause the death of MSC, the inflammatory environment can also increase the expression of HLA-DR (MHC Class II antigen) antigen of MSC, enhance the immunogenicity of MSC, and be recognized by immune cells, accelerating the clearance of MSC.
The main sources of mesenchymal stem cells (MSC) are the umbilical cord and placenta of newborns and adult fat. Because there is no immune rejection, proliferation and differentiation ability, it has become a "star of hope" in regenerative medicine, playing an increasingly important role in disease treatment and damage repair.
MSC has multiple functions, such as immunosuppressive function, promoting the repair function of body tissues and organs, promoting angiogenesis/regeneration, and supporting the proliferation and differentiation of hematopoietic stem cells, so MSC has a wide range of clinical indications.