
Source: Stem cell base
Since the National DRUG Administration's CENTER FOR DRUG EVALUATION (NMPA) re-accepted the application for clinical trials of stem cell new drugs in 2018, the application for clinical trials of stem cell drugs has steadily increased. The total number of acceptance numbers has increased from 5 declared in 2018 (in terms of acceptance numbers) to 47 in 2023, nearly doubling from 2022, and the speed of research and development of new stem cell drugs in China has significantly accelerated. The number of stem cell drug applications in 2020-2023 is shown in Figure 1.
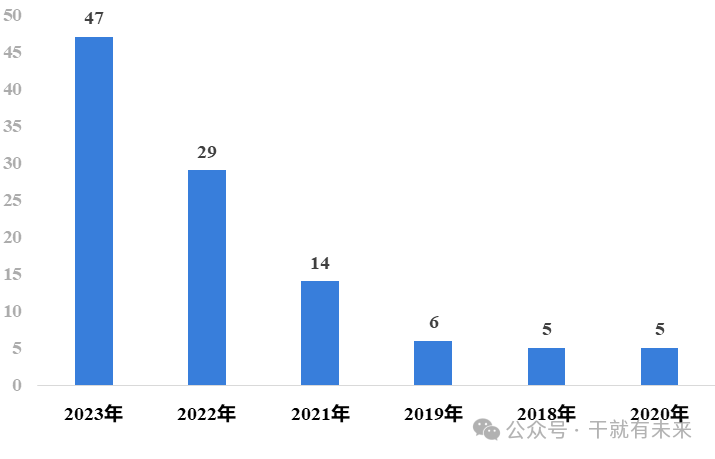
Figure 1 Number of stem cell drug applications in 2020-2023
1. Declaration
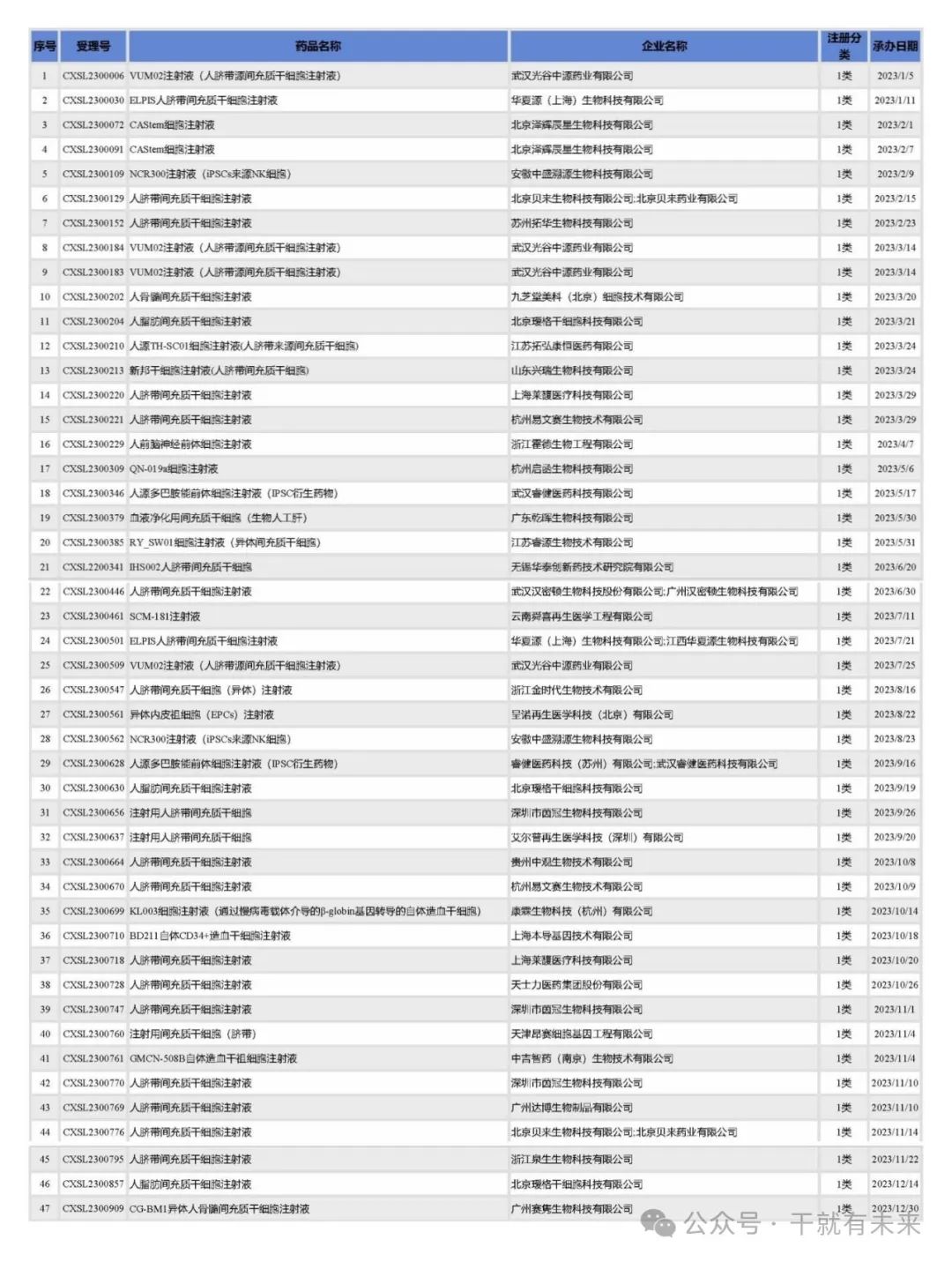
In 2023, CDE accepted 47 clinical applications for stem cell drugs (counted by acceptance number), and the registration classification was all domestic category 1 innovative drug registration applications, as shown in Table 1.
Table 1 List of acceptance of stem cell drug registration applications in 2023
二、Clinical trial implied approval
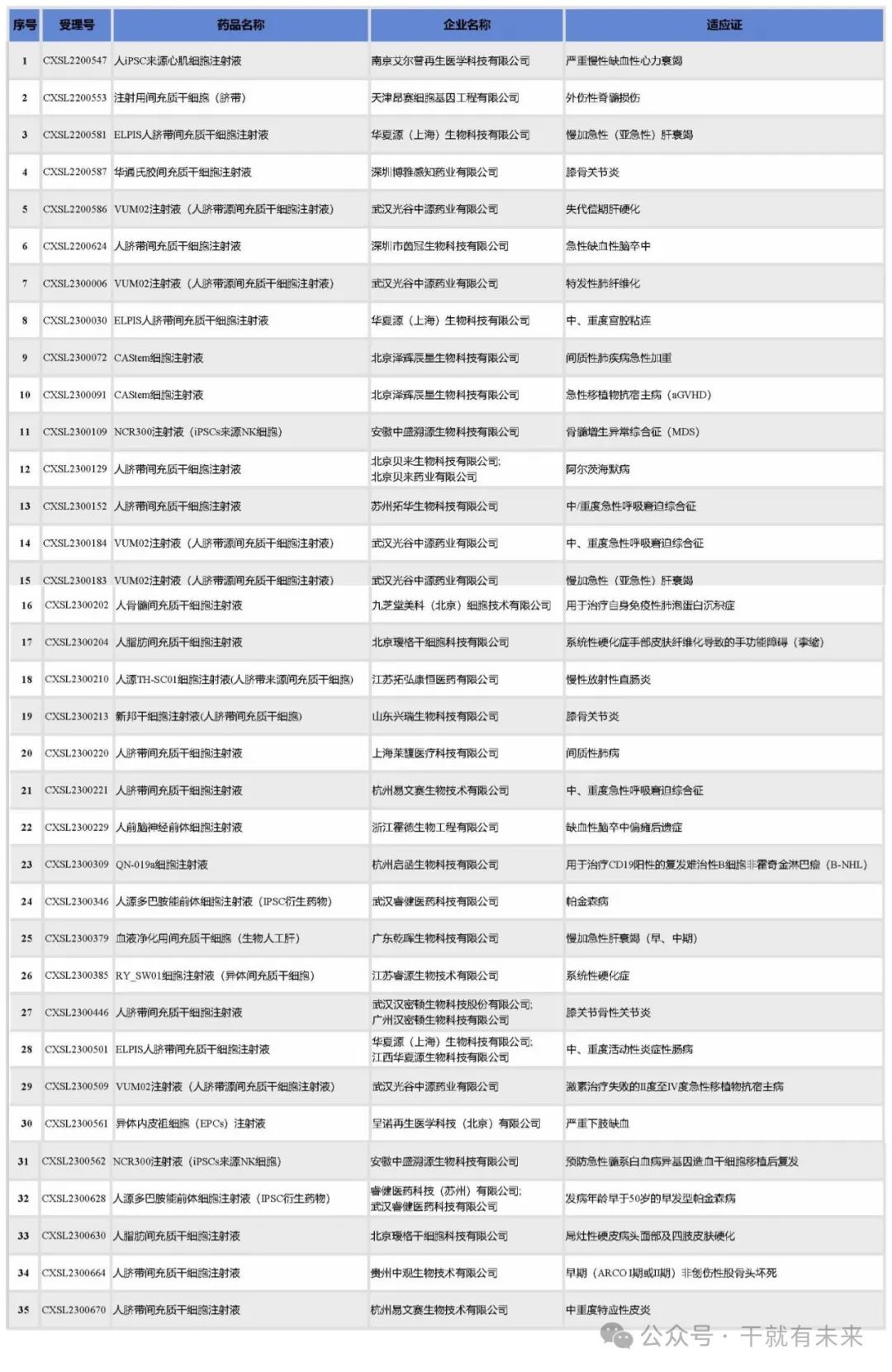
In 2023, CDE acquiesced in the clinical trials of 35 stem cell drugs from 21 companies. Among them, Wuhan Guanggu Zhongyuan Pharmaceutical Co., LTD. 's human umbilical cord derived mesenchymal stem cell injection was approved for 5 clinical trial implied licenses, and Beijing Zehui Chenxing Biotechnology Co., LTD., Anhui Zhongsheng Xuyuan Biotechnology Co., LTD., Beijing Aige Stem Cell Technology Co., LTD., Hangzhou Yiwensai Biotechnology Co., Ltd. was approved for 2 clinical trial implied licenses each, as shown in Table 2.
Table 2 List of implied approvals for clinical trials of stem cell drugs in 2023
三、Clinical trial indication analysis
As of December 31, 2023, a total of 35 clinical trials approved by CDE involved 31 indications. These include lung disease (7 items), Alzheimer's disease, liver failure, knee osteoarthritis, chronic radiation proctitis and so on. From the perspective of indications, the vast majority of drugs utilize the immune regulation ability of mesenchymal stem cells to treat diseases related to inflammation or the autoimmune system.
四、Declared cell type analysis
As of December 31, 2023, according to the declared cell types, mesenchymal stem cell drugs totaled 60 types, accounting for 75.9%, which is the most important cell type for stem cell drug development, as shown in Figure 2. In particular, in 2023, a total of 21 umbilical cord mesenchymal stem cell drugs were accepted, ushering in a significant increase. Moreover, in addition to mesenchymal stem cells, potent cell drugs derived from human induced pluripotent stem cells (IPscs) also showed a steady growth trend, and 4 new IPSC-differentiated functional cell drugs were introduced in 2023.
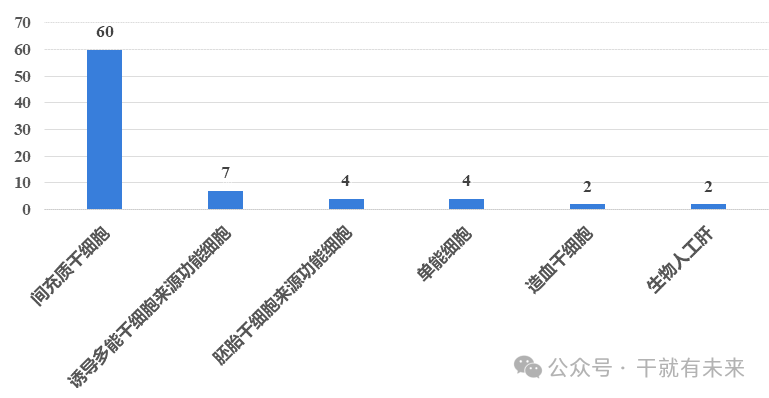
Figure 2 Cell types of IND stem cell drugs approved
五、Analysis of the situation in the reporting area
In terms of the application of stem cell drugs in various provinces and cities, as of December 31, 2023, a total of 106 applications were accepted, and 79 applications for implied approval of clinical trials. In terms of application acceptance, Shanghai ranked first with 21 items, followed by Beijing with 18 items, followed by Guangdong, Jiangsu and Zhejiang, as shown in Figure 3.
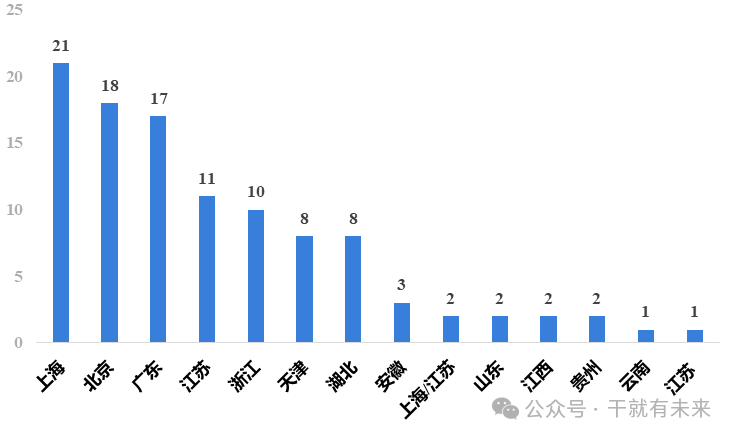
Figure 3 Acceptance of stem cell drug applications by provinces and cities
In terms of implied approvals for clinical trials, Shanghai ranked first with 17, followed by Beijing with 16, followed by Guangdong, Jiangsu and Hubei. It can be seen that Shanghai is the most active area for stem cell drug research and development, and the Yangtze River Delta, Beijing and Guangdong are the main areas for stem cell drug research and development in China, as shown in Figure 4.
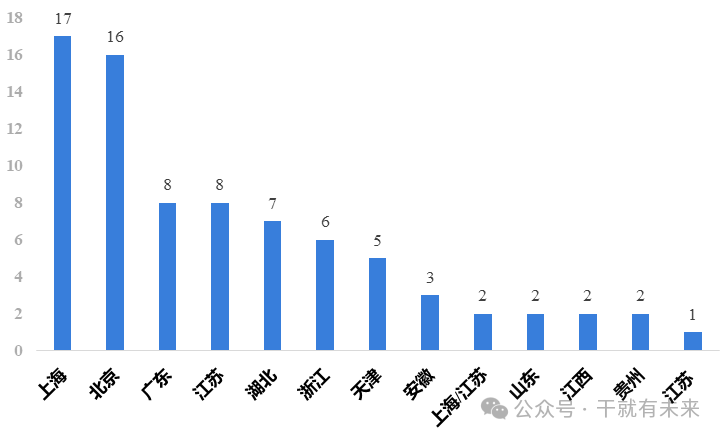
Figure 4 Implied approval of clinical trials of stem cell drugs in provinces and cities
六、2024 stem cell drug filings may usher in an even bigger explosion
The global field of cell therapy has made many major original achievements in the field of basic research. The basic research of stem cells in China ranks among the top in the world, providing new hope for the treatment of many difficult diseases. However, due to the disorderly development of the early market, China's stem cell clinical research and clinical trials once stalled, resulting in the current stem cell industry upstream storage business is relatively mature, midstream development and downstream application development is relatively lagging behind the situation.
With the "Management Measures for Stem Cell Clinical Research (Trial)" and the "Guiding Principles for Quality Control and Preclinical Research of Stem Cell Preparations (trial)" "Technical Guidelines for Research and Evaluation of cell therapy products (trial)" "Technical Guidelines for non-clinical research of Gene-modified cell therapy products (trial)" "Technical Guidelines for pharmaceutical research and evaluation of human stem cell products (trial)" "Technical Guidelines for clinical trials of human stem cells and their derived cell therapy products (trial)" "Cell and gene therapy products Policies such as the Guidelines for Bed-related Communication Technology have been introduced, and China's clinical transformation of stem cells has gradually moved on the right track, and gradually clarified the road to using stem cell drugs as transformation exports.
With the gradual clarity of the policy, after some exploration, the clinical transformation of stem cells in China has entered a stage of orderly development, and the quantity and quality of stem cell drug declarations have increased significantly. According to the world's largest clinical trial registration website http://clinicaltrials.gov query information, such as new crown infection sequela, diabetes, myelodysplastic syndrome, severe neonatal hypoxic ischemic encephalopathy, peyronie's disease, erectile dysfunction and other diseases of clinical research is carried out in application of stem cell therapy. It is believed that the year 2024 will be an explosion period of stem cell drug research and development, and more stem cell drugs for new indications will enter the clinical trial stage. At the same time, the phase I/II clinical trials of stem cell drugs are being carried out and will gradually produce results. It is believed that with the continuous development of stem cell technology, stem cells as therapeutic drugs can be listed in the future.