
The field of cell and gene therapy (CGT) has a bright future, and the global CGT therapy will still shine in 2024.
According to statistics, from January to April 2024, a total of 5 CGT therapies were approved to be put on the market, including 3 cell therapies and 2 gene therapies.
Lovance:Amtagvi.
On February 17th, Iovance Biotherapeutics announced that FDA in the United States has accelerated the approval of its tumor infiltrating Lymphocyte (TIL) therapy Amtagvi (lifileucel) for the treatment of advanced melanoma, priced at $515000.
According to the press release, lifileucel is the world's first approved TIL cell therapy. Amtagvi is a tumor-derived autologous T cell immunotherapy. By obtaining tumor tissue from the patient and extracting TIL, and then using IL-2 cytokines in vitro to stimulate TIL amplification. This in vitro stimulation not only increases the number of TIL, but also activates the anti-tumor ability of TIL. These TIL are then injected back into the patient's body to kill tumor cells more effectively.
Lifileucel showed clinically significant anti-tumor activity, and the median duration of remission (DOR) of patients has not yet been achieved (95% CI:8.3- has not yet been achieved). The overall remission rate of all patients with advanced PD-1/PD-L1 drug-resistant melanoma (nude 153) who received a single injection of lifileucel was 21.9% in the fourth year, and the median overall survival time was 13.9 months (95% CI:10.6-17.8).
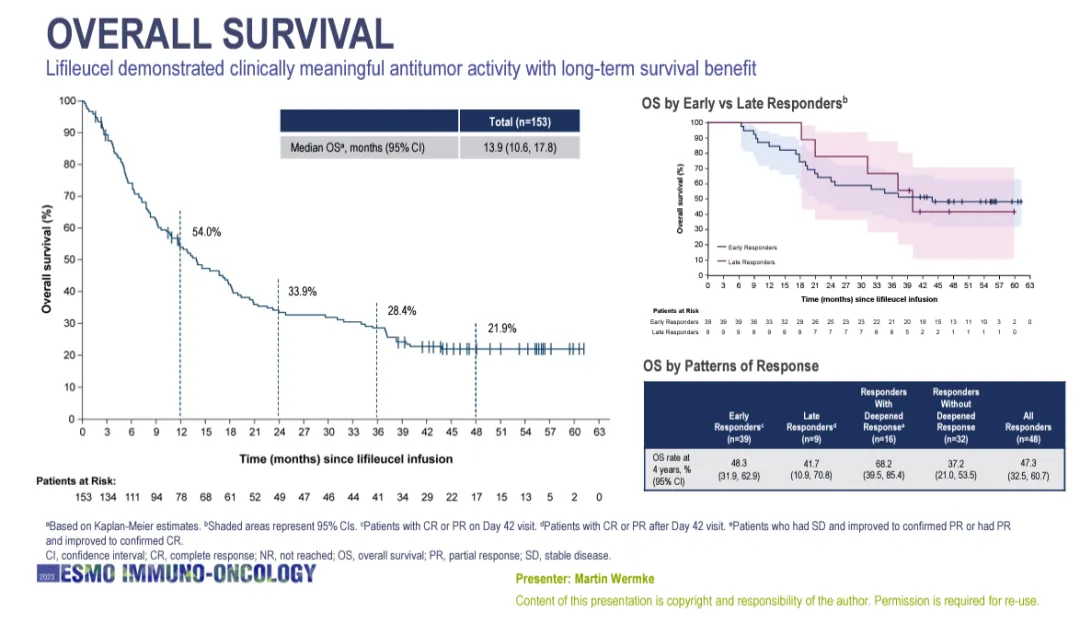
Overall survival data of Lifileucel at a median follow-up of 4 years
Iovance Biotherapeutics is a leader in the field of TIL cell therapy. In addition to LN-145-S1 (PD-1 selective TIL therapy) and LN-145-Gen 3 (third generation TIL therapy) in LN-144 and LN-145,lovance pipelines, they have also entered phase 2 or phase 3 clinical trials, involving indications such as head and neck squamous cell carcinoma (HNSCC) and metastatic non-small cell lung cancer (NSCLC).
Keji Pharmaceutical Co., Ltd.: Zevoji Orensai injection.
On March 1st, Keji Pharmaceutical announced that the State Drug Administration (NMPA) has approved the application for a new drug (NDA) of its BCMA CAR-T product Sekezer ®(Zevoji Orenser injection) for the treatment of adult patients with recurrent or refractory multiple myeloma who have progressed after at least 3 lines of treatment (with at least one proteasome inhibitor and immunomodulator). This injection is the fifth approved CAR-T therapy in China, and it is also the second CAR-T product targeting BCMA in China.
On March 5, Keji Pharmaceutical revealed that the initial price of the injection (trade name: Saikaize) was 1.15 million yuan. According to joint estimates with partner East China Pharmaceutical (000963.SZ), the terminal sales of the injection were expected to reach a peak of more than 1 billion yuan. Zevogi Orenser is an autologous BCMA targeting CAR-T cell product, which is produced by lentivirus transduction of T cells. The CAR encoded by lentivirus includes human BCMA specific single strand variable fragment (scFv), human CD8 α hinge domain, CD8 α transmembrane domain, 4-1 BB costimulatory domain and CD3 ζ activation domain. The new all-human scFv developed by ourselves has high binding affinity and stability.
BMS:Breyanzi.
On March 14, Bristol-Myers Squibb (Bristol Myers Squibb) announced that FDA in the United States has accelerated the approval of Breyanzi (lisocabtagene maraleucel;liso-cel). This is a CD19-targeted CAR-T cell therapy for adult patients with relapsed / refractory chronic lymphoblastic leukemia (R CLL) or small lymphocytic lymphoma (SLL) who have previously received at least two lines of treatment, including BTK inhibitors and BCL-2 inhibitors. According to the press release, this cell therapy is the first CAR-T cell therapy for adult patients with recurrent or refractory CLL or SLL. Priced at $410000 per dose, Breyanzi is an autologous CAR-T cell therapy targeting CD19 antigen with a clear composition and 4-1BB costimulatory domain.
It was approved by FDA in February 2021 for the treatment of adult patients with recurrent / refractory large B-cell lymphoma (LBCL) who have received two or more systematic treatments. What is unique about this treatment is that the proportion of CD8-positive and CD4-positive T cells in CAR-T therapy is controlled, thus the toxicity and side effects of cell therapy can be better controlled. The 4-1BB signal domain enhanced the expansion and persistence of CAR-T cells. The TRANSCEND CLL 004 trial based on this approval is an open-label, single-arm, multicenter, phase 1, phase 2 clinical trial. Data analysis showed that the trial reached the main end point, 20% of patients with recurrent or refractory CLL or SLL who received Breyanzi treatment achieved complete remission (CR), and safety was confirmed.
Orchard Therapeutics:Lenmeldy.
On March 18, FDA of the United States approved the gene therapy Lenmeldy of the British company Orchard Therapeutics for the treatment of children with heterochromatic leukodystrophy (MLD). The drug is priced at $4.25 million, or about 30.6 million yuan, surpassing Hemgenix ($3.5 million) to become the most expensive drug in history, setting another record for "sky-high prices". MLD is caused by a mutation in the arylsulfatase-a (ARSA) gene, which causes sulfate to accumulate in the brain and other areas of the body, such as the liver, gallbladder, kidney, and spleen.
Over time, the patient's nervous system is damaged, leading to motor, behavioral and cognitive degradation, as well as severe spasms and seizures. Patients will gradually lose the ability to move, speak, swallow, eat and so on. Lenmeldy transduced ARSA gene encoding arylsulfase-An into CD34-positive hematopoietic stem cells collected from MLD patients by lentiviral vector to restore the expression of arylsulfase-A, and then transfusion of hematopoietic stem cells into the patient to achieve the effect of one-time treatment of the disease.
Pfizer: BEQVEZ.
On April 26th, Pfizer (Pfizer) announced that FDA in the United States has approved BEQVEZ (fidanacogene elaparvovec-DZKT), a gene therapy based on adeno-associated virus (AAV) vector, for the treatment of adult patients with moderate to severe hemophilia B and negative neutralizing antibodies to AAV serotype Rh74 (18 years old and over).
It is reported that the price of BEQVEZ is 3.5 million US dollars, which is in line with the price of Hemgenix, a type B hemophilia gene therapy developed by UniQure, which was approved to go on the market. Previously, Health Canada approved the listing of Beqvez in Canada on January 3, 2024.
BEQVEZ is a kind of gene therapy drug in vivo, which uses genetically engineered AAV capsid to improve the ability to deliver highly active human FIX gene to human target tissues and organs. Beqvez aims to enable patients to produce enough FIX protein on their own, so that they do not need to receive regular FIX infusion, so as to improve patients' blood coagulation function. The mode of administration is intravenous infusion, and one administration is effective for a long time or even for a lifetime.
Summary.
The CGT field is one of the areas where sky-high drugs occur frequently. This year, Orchard Therapeutics's Lenmeldy and Pfizer's Beqvez hit the list of the most expensive drugs, occupying the first place and tied for the second place respectively.
Looking to the future, more potential cell and gene therapy are constantly developing and diversifying. No matter from the treatment concept, global promotion, and the degree of attention at the national level, the field of CGT is undoubtedly an area with long-term technological breakthrough needs, so further research and practice are needed to solve the challenges and problems, and to ensure the safety and effectiveness of cell and gene therapy.
In 2024, seven other CGT therapies are expected to be approved and put on the market, including Hengrun Dasheng's CAR-T cell therapy product Rundaji Orenser injection, Adaptimmune Therapeutics's engineered T-cell therapy Eki Olensai, BioCardia's CardiAMP, Pfizer's new research gene therapy Fidanacogene elaparvovec, Rocket Pharmaceuticals's gene therapy Kresladi, Autolus Therapeutics's CAR-T cell therapy Obecabtagene autoleucel, Abeona Therapeutics's ongoing autologous cell therapy Prademagene zamikeracel (pz-cel).