
Recently, IQVIA, a global company in the field of life sciences, released a research report on the current status and future trends of cell and gene therapy.
The report notes that cell and gene therapy (CGT) has brought revolutionary benefits to patients who lack other treatment options, and that investment in the field, clinical research and new product launches have increased significantly in recent years. At the same time, the report also makes an in-depth analysis of eight key factors affecting the future development of this field.
Next, this article will introduce the core content of this report in detail, so that readers can better understand the latest progress and development trends in this field.
According to the IQVIA report, the volume of transactions in the field of cells and gene therapy has increased by 48% compared with 10 years ago, and the proportion of transactions in the life sciences as a whole has increased year by year. In particular, in 2023, the share of transactions in this sector has exceeded 10 per cent, compared with only 5 per cent in 2014. These data fully reflect the industry's confidence and interest in cell and gene therapy models.
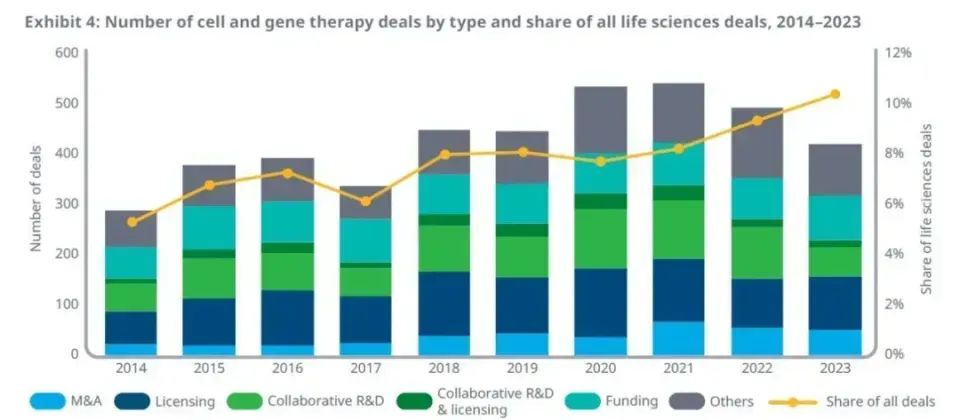
The number of transactions in the field of cell and gene therapy and their share of all life science transactions have increased significantly over the past decade
Through continuous efforts and innovation, clinical development activities in the industry have reached a new peak. A total of 3285 clinical trials have been launched in the past five years to assess the role of cell and gene therapy in different types of patients, the report said. In 2023, 631 clinical trials were launched. Among them, clinical trials initiated by biomedical companies accounted for 64%. The number of clinical trials initiated by the industry has increased significantly over the past decade, up 276% from 2013, 34% from five years ago, and reaching a new high in 2023.
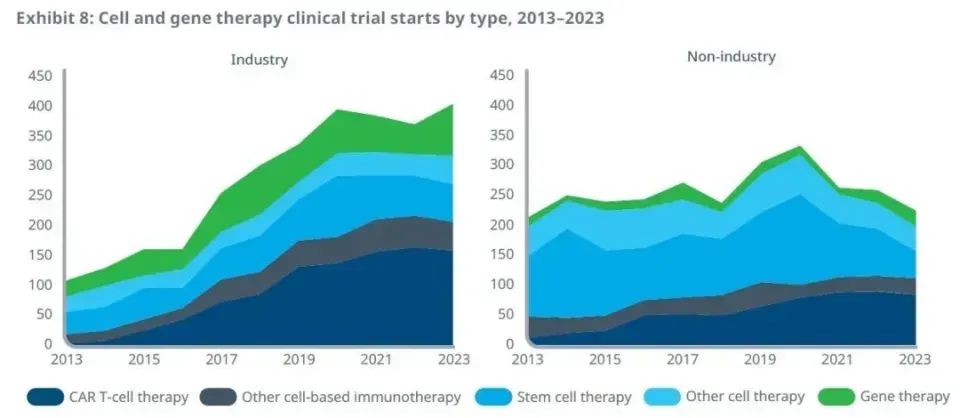
The increase in these clinical trials mainly comes from clinical trials of CAR-T cell therapy. It is worth mentioning that in 2023, the proportion of clinical trials of CAR-T therapy initiated by biomedical companies decreased compared with 2022, while the proportion of gene therapy increased to 22% from 14% in 2022, showing signs of a shift in the focus of the industry.
In the field of treatment, the main treatment area of CAR-T therapy is still hematological cancer and solid tumor, but although there are few clinical trials targeting autoimmune diseases, recent research results show the potential of CAR-T therapy in this field. In a study published in February in the New England Journal of Medicine (doi: 10.1056/NEJMoa2308917), CD19 targeted CAR-T cell therapy to relieve 100% of autoimmune patients and continue to stop using immunosuppressive drugs. Patients in the study included patients with systemic lupus erythematosus, idiopathic inflammatory myositis and systemic sclerosis.
The areas of disease treated by gene therapy are more diversified, with clinical trials for the treatment of solid tumors accounting for 35%. In addition, there are significant clinical development activities in the fields of neurological, metabolic, endocrine, cardiovascular and ophthalmic diseases. Gene therapy is often used to treat genetic diseases and to give hope to patients who may face the threat of permanent disability. For example, earlier this year, research teams from Lilly and Fudan University reported the results of clinical trials of their respective gene therapy for hereditary deafness in children. In two clinical trials, the hearing of children treated with gene therapy was significantly improved.
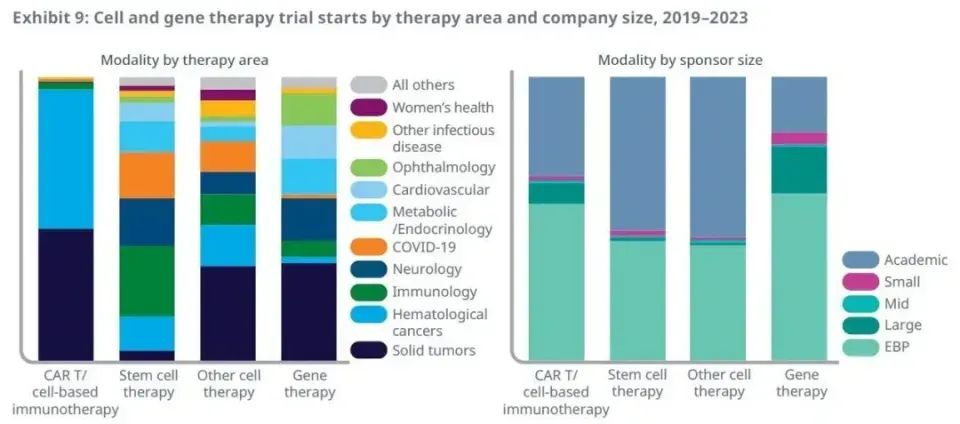
Clinical trials initiated by large pharmaceutical companies account for 6% of the total, with the vast majority focusing on the field of cell and gene therapy. As global regulators accelerate the launch of products, the number of approved treatments has doubled.
By the end of 2023, 76 cell and gene therapies had been approved by regulators worldwide, doubling the total number in 2013, according to the report. In the past three years, 20 new cell and gene therapies have been successfully approved.
Among these approved treatments, CAR-T therapy and other cell-based cancer immunotherapies have made significant progress over the past decade, with 12 related treatments approved by the end of 2023. At the same time, a total of 19 gene therapies have been approved worldwide, mainly for the treatment of various genetic diseases.
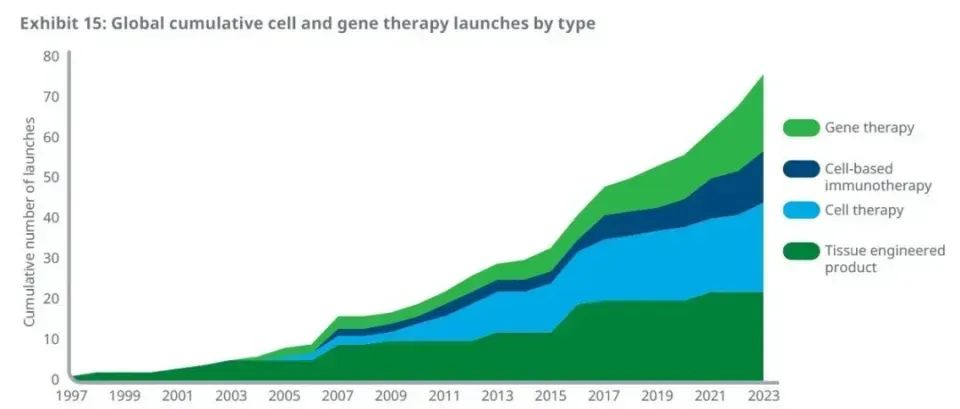
Number and types of approved cell and gene therapy worldwide
In 2023, a number of approved cell and gene therapies represent a major breakthrough in the field of disease. For example, the first cell therapy to treat type 1 diabetes has been approved. The first gene therapy for Duchenne muscular dystrophy has been approved. In 2023, the first repeatable gene therapy was also approved by FDA in the United States.
In the area of regulatory review, in view of the increasing range of cell and gene therapy research and development activities, global regulators are actively taking new measures to promote the promotion and evaluation efficiency of R & D projects. In the United States, for example, according to the IQVIA report, 86% of projects approved for cell and gene therapy in the past five years have received at least one qualification for accelerated research and development of FDA. Of these, 71% of treatments were awarded breakthrough therapy, while 64% of treatments obtained fast-track qualifications, significantly exceeding the proportion of acellular and gene therapy. These data fully show that cell and gene therapy is usually targeted at areas of disease that are serious and significantly do not meet medical needs.
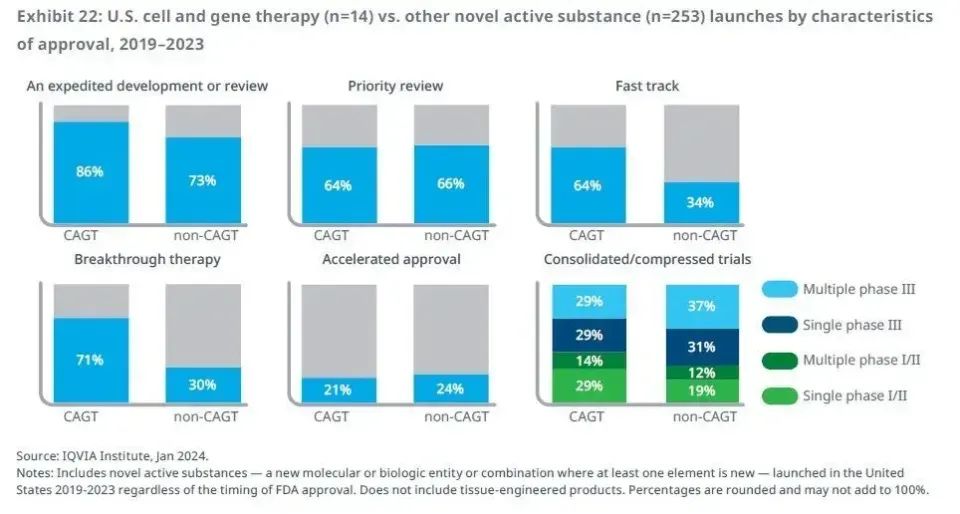
In addition, the United States FDA also launched the Advanced Therapy of Regenerative Medicine (RMAT) recognition system. For projects that meet this recognition, FDA will provide support for both fast-track eligibility and breakthrough therapy recognition.
Globally, other regulators have also provided accelerated channels for the development of cell and gene therapy, such as the PRIME identification of the European Drug Administration (EMA), accelerated evaluation and conditional listing, priority review and conditional approval by Australian regulators, and SAKIGAKE approval by Japanese regulators.
The Future of Cell and Gene Therapy.
The report points out that cell and gene therapy have made great progress in the past decade, but the industry is still in its immature stage compared with the field of small molecular drugs and antibodies, and there is still a lot of uncertainty about its future development.
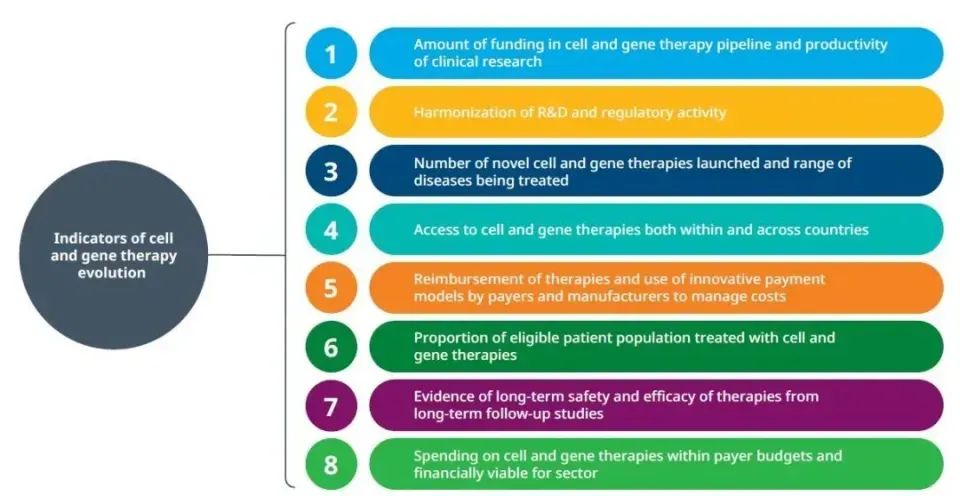
The future development of the cell and gene therapy industry depends on the progress of eight indicators, such as product research and development activities, regulatory approval, patient accessibility, long-term efficacy and safety of therapy.
In 2023, the number of approvals for cell and gene therapy reached an unprecedented high. The report predicts that with the continuous optimization of the regulatory review process and the potential of cell and gene therapy applications outside oncology, more than 12 cell and gene therapy products are expected to be approved annually in the future. Of these, more than half of the treatments are expected to be used in diseases other than oncology, which is expected to bring good news to more than 10,000 patients.
At present, cell and gene therapy are receiving more and more attention and in-depth research, and people are optimistic and confident about their potential and prospects in the medical field. The initial achievements in the field of cell and gene therapy and their continued success in clinical practice have brought opportunities for many large pharmaceutical companies to attract a large amount of investment, thus promoting continuous innovation in this field.