
By the end of April (the first quarter of 2024), seven new drugs for mesenchymal stem cells (excluding hematopoietic stem cells), IND, have been approved by the Drug Evaluation Center (NMPA) of the State Drug Administration, which involves the prevention and treatment of stroke, diabetic foot, chronic obstructive pulmonary disease, ulcerative colitis, chronic heart failure and other chronic diseases.
Up to now, a total of 63 new drugs for mesenchymal stem cells (IND) have been approved in China. With the continuous increase in the number of stem cell clinical trials, it is bound to promote the vigorous development of China's stem cell industry!
How much do you know about stem cells and mesenchymal stem cells?
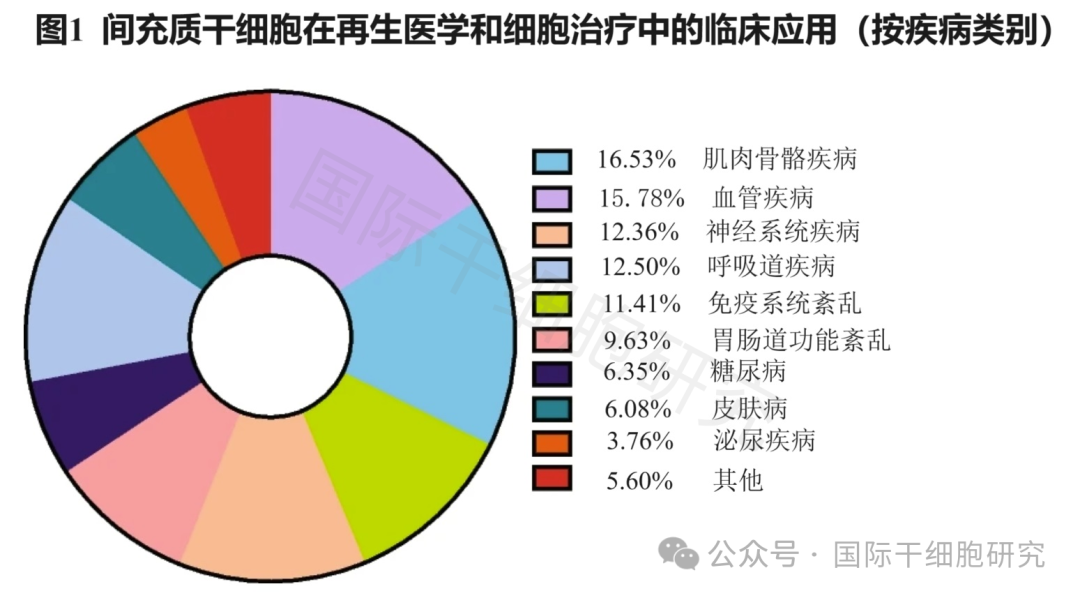
Stem cells are a kind of "primitive cells" that can differentiate into a variety of tissue cell types and have the ability of self-renewal, including embryonic stem cells, induced pluripotent stem cells, mesenchymal stem cells and many other family members. In particular, "mesenchymal stem cells" are more widely used in clinic.
Among them, mesenchymal stem cells (MSC) are a kind of pluripotent stem cells derived from mesoderm, which have multiple functions such as tissue repair, immune regulation, proliferation and differentiation potential, angiogenesis and so on. They can be isolated from many tissues and organs of the body, such as umbilical cord, adipose tissue, bone marrow, dental pulp, etc., and have unique advantages in the prevention and treatment of chronic diseases, cosmetology and anti-aging, and enhancing immunity. Therefore, it is also known as the "all-around player" of stem cells!
Stem Cell Regulatory Policy and New Drug approval process in China.
Regulatory Policy of Stem Cell Therapy in China.
Since the "notice on doing well the Supervision and Management of Stem Cell Clinical Research in 2019" was issued in 2019, China has begun to adopt the "quasi-dual-track management mode" of stem cells, which is jointly supervised by the State Drug Administration and the "Health Commission". In order to establish stem cell technical standards in line with our own national conditions.
During 2022-2023, China successively promulgated a number of laws and regulations, including "guidelines for the Application of key projects of Stem Cell Research and Organ repair in 2022 (draft for soliciting opinions)" and "guidelines for production quality Management of Cell Therapy products (trial)", in order to further supervise the clinical trials, clinical application, production management and quality management of stem cells and their related products.
Approval process of new stem cell drugs.
From research and development to approval and marketing, a new drug usually needs to go through the necessary stages such as preclinical research, phase 3 clinical trials, new drug IND and NDA application, and so on. Only when these approval processes are passed smoothly can a new drug be approved for market, and new stem cell drugs are no exception. Many patients may have heard of "new drugs IND and NDA", but do not understand its meaning and importance, the following is a brief introduction for reference.
1、Application for Clinical Research of New drugs (IND).
When a stem cell product or a new clinical drug successfully passes the preclinical trial, an application for clinical research of the new drug needs to be submitted to the US Food and Drug Administration (FDA) or the State Drug Administration (China). If the FDA does not reject the application within 30 days after submitting the application, the clinical research application for the product is deemed to be valid and relevant clinical trials can be conducted.
It is reported that a stem cell product called "JadiCell" has submitted a Phase III clinical trial application (Clinical Trials Arena) to FDA in the United States, looking forward to the good news that the product will be launched as soon as possible.
2、New Drug Application (NDA).
After completing all the phase 3 clinical trials, the product can collate and summarize the research data and data, and then submit a new drug application (NDA) to the US FDA (or the FDA of China) to ensure the effectiveness, safety and quality control of the drugs on the market.
FDA usually completes the NDA approval of the product within a specified period of 6 months. But usually, due to too many application materials, the approval of many new drugs may take longer.
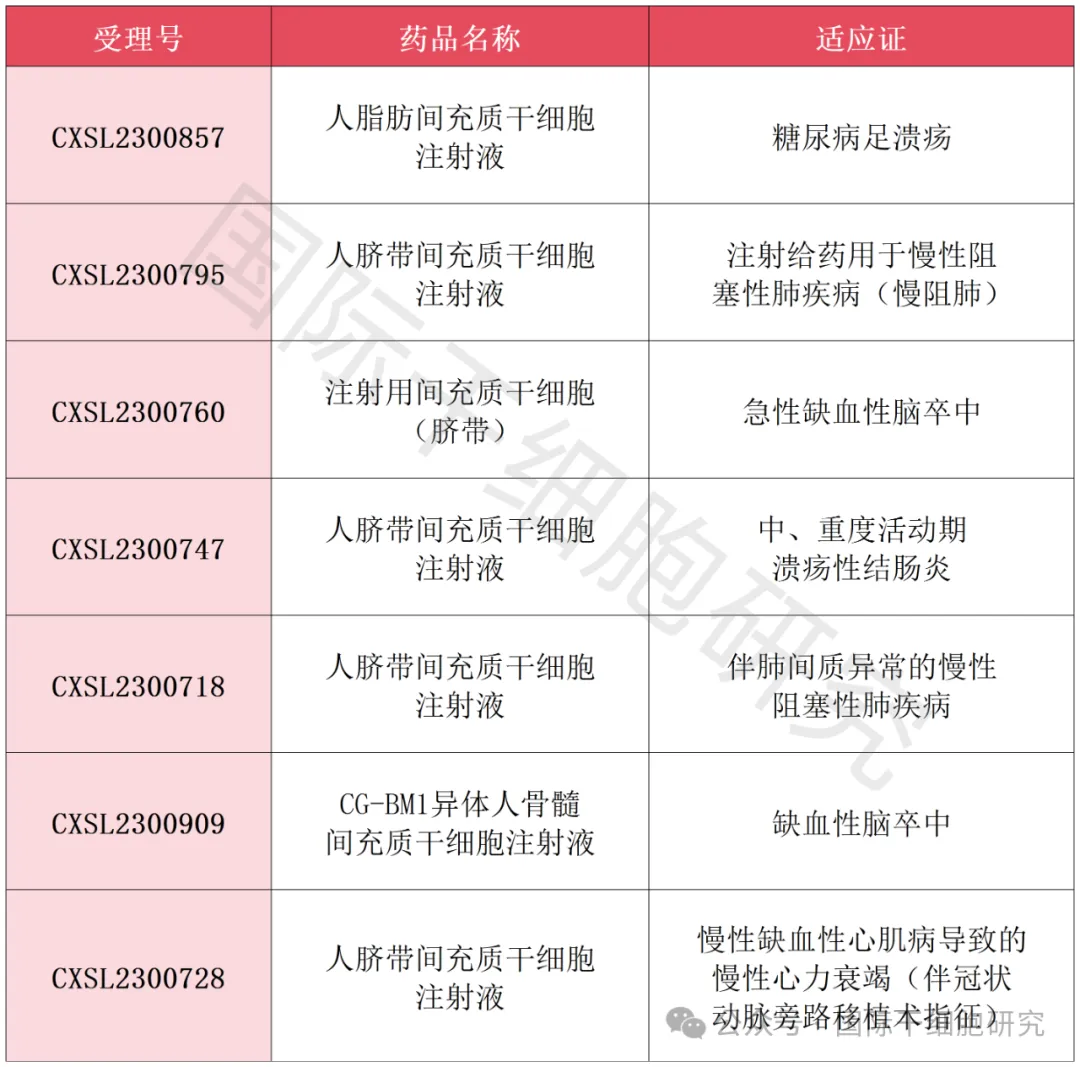
New stem cell IND in the first quarter of 2024.
By the end of April 2024, there are seven new drugs for mesenchymal stem cells (except hematopoietic stem cells) IND in China, which have been approved by the Drug Evaluation Center (NMPA) of the State Drug Administration. Here is a brief summary for your reference (see table below).
The editor sent a message:
Mesenchymal stem cells have good clinical safety, multi-directional differentiation potential, immune regulation and self-renewal ability, and are rich in sources. many types of clinical applications, including umbilical cord mesenchymal stem cells, dental pulp mesenchymal stem cells, adipose mesenchymal stem cells and so on, have irreplaceable advantages in the prevention and treatment of chronic diseases, cosmetology and anti-aging.
What is gratifying is that in recent years, the clinical transformation of stem cells in China has shown a trend of vigorous development, and the new drug IND has increased year by year, covering a wider range of diseases. The editor also hopes that with the continuous expansion of the research and development scale of stem cell products, our regenerative medical technology can catch up with stem cell powers such as the United States and Japan as soon as possible for the benefit of more patients!
Reference:
[1]Maldonado,et al.Clinical utility of mesenchymal stem/stromal cells in regenerative medicine and cellular therapy.J Biol Eng 17,44(2023).
https://jbioleng.biomedcentral.com/articles/10.1186/s13036-023-00361-9#citeas
[2]https://www.cde.org.cn/main/xxgk/listpage/4b5255eb0a84820cef4ca3e8b6bbe20c