With the continuous breakthrough of cell therapy and gene therapy technology, it has an increasingly broad application prospect in the field of disease treatment. However, the application of any innovative technology is inseparable from scientific and standardized supervision and guidance. In recent years, relevant state departments have issued a series of laws and regulations and guiding principles to promote the healthy development of this field. The purpose of this paper is to summarize these regulations, to help readers understand the regulatory framework in the field of cell therapy and gene therapy, and to provide help for related research and clinical practice.
Regulations on cell and gene therapy
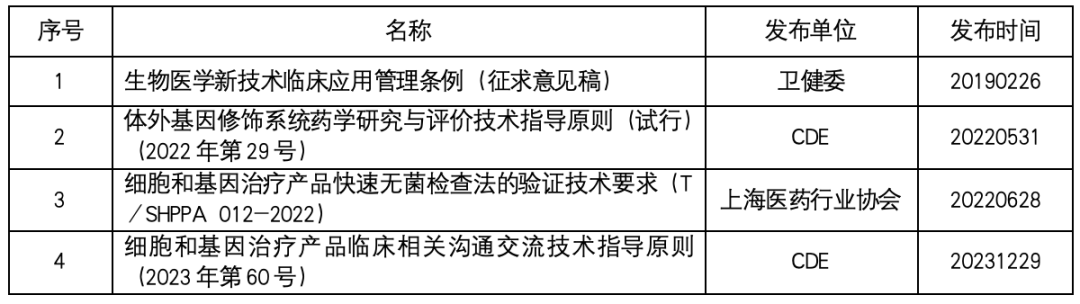
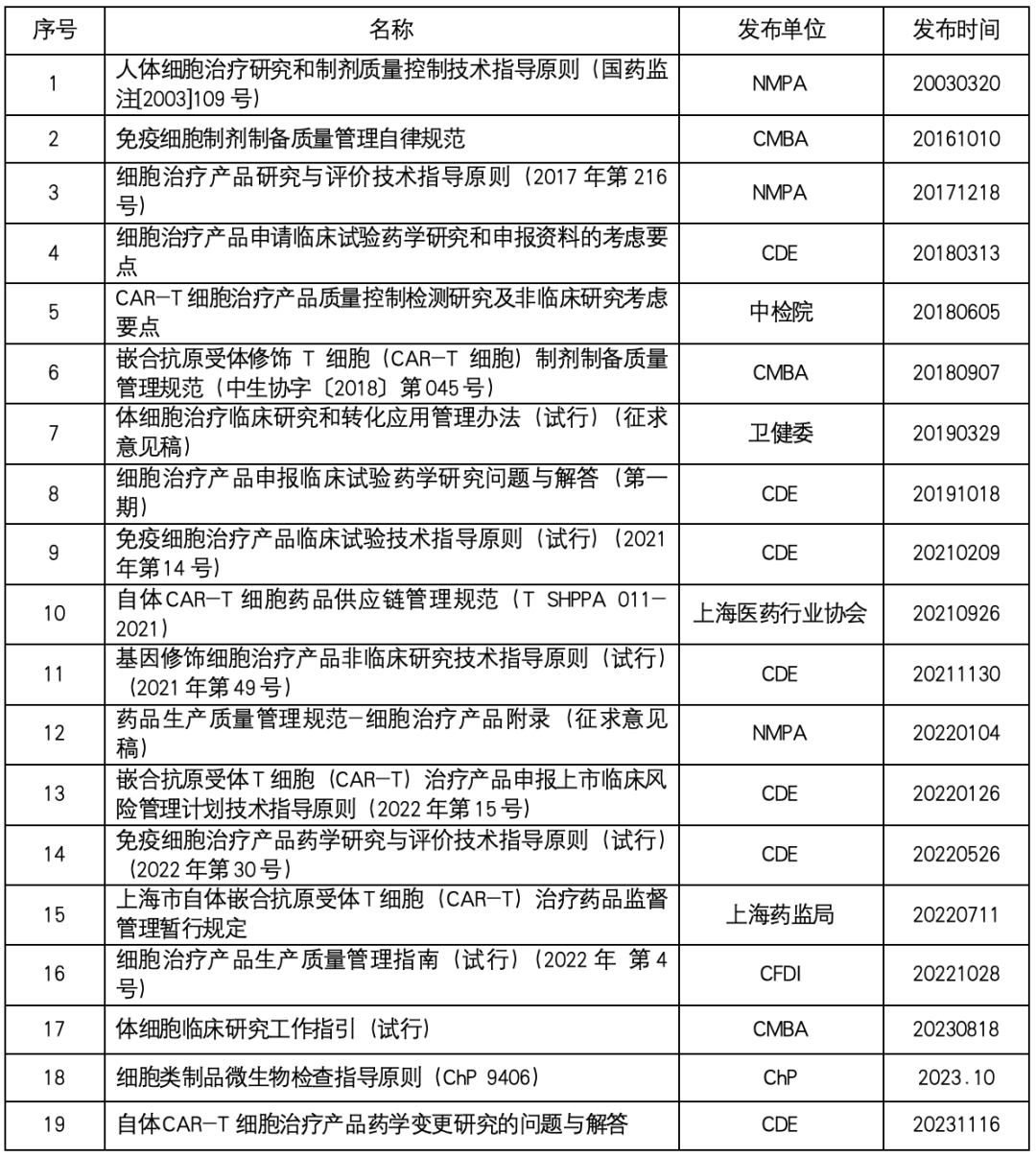
Cell therapy laws and regulations
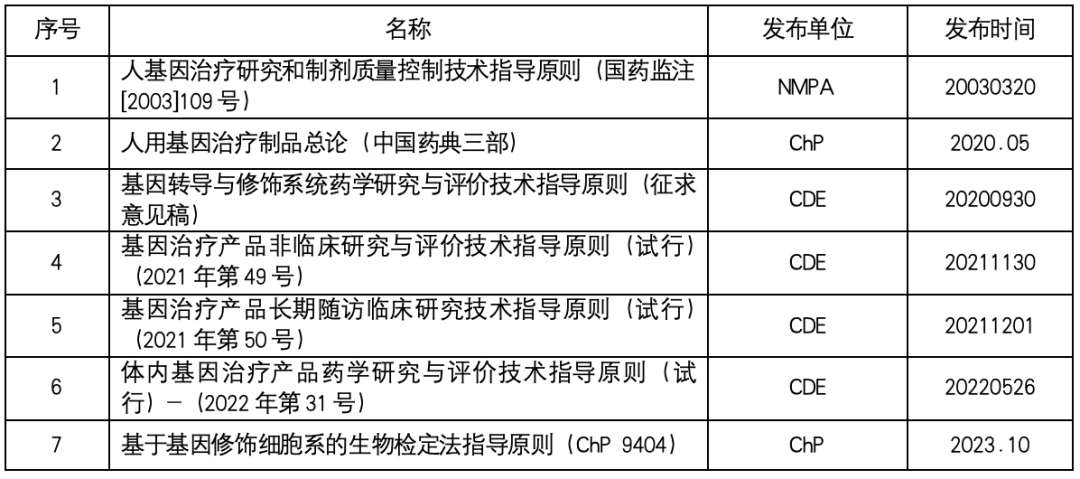
Gene therapy laws and regulations
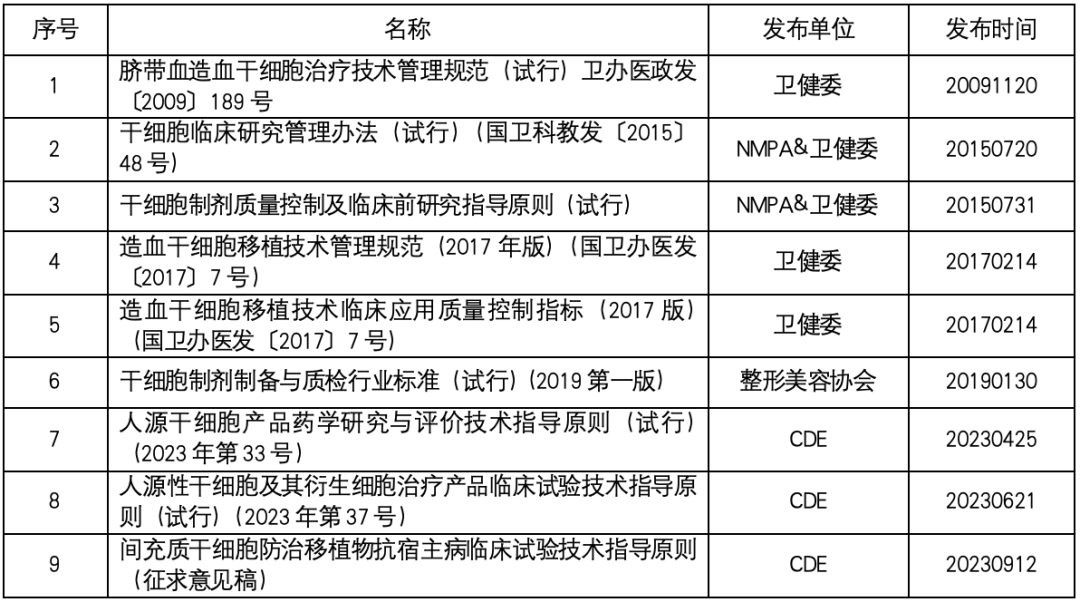
Stem cell regulation