
Cell therapy is one of the popular tracks for innovative medicine in China. Halfway through 2024, Yimaike has counted the clinical application progress of domestic cell therapy from January to June this year. A total of 54 companies have achieved new results, involving 57 cell therapy drugs, including stem cells, CAR-T, TIL, TCR-T, NK, T cells, γδ-T, macrophages, STAR-T, CAR-DNT, CAR-raNK, etc. Among them, stem cells and CAR-T are the main ones, and the types are diverse. Some unique immune cells have also been developed for cancer treatment. It is gradually developing towards a trend of "letting a hundred flowers bloom"(the statistical part is for new drug applications for biological products. If there are any omissions, please add them).
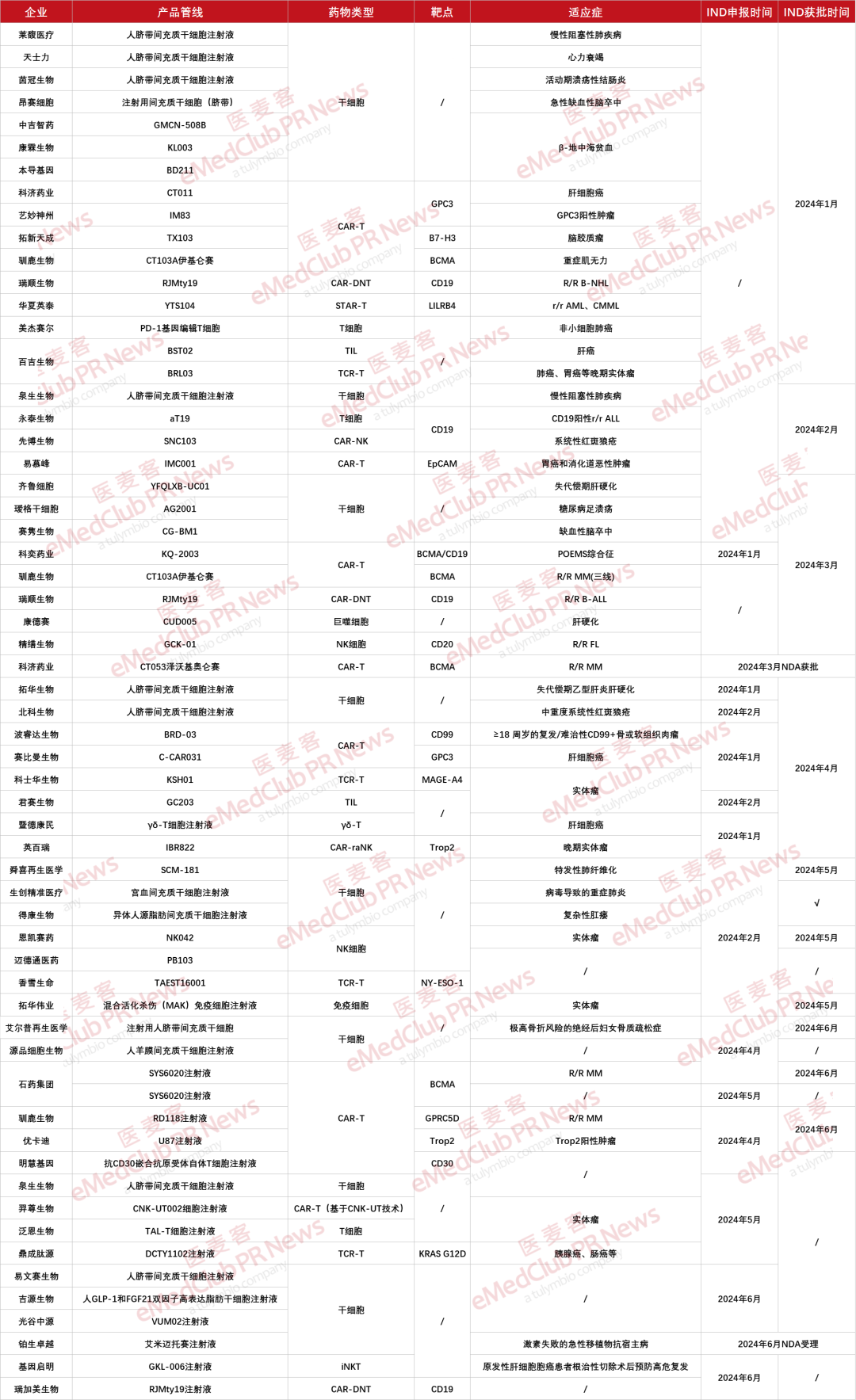
▲ Progress in clinical application for H1 cell therapy in 2024
Among these candidate products, there are 45 IND approval records (the/form has not been found for a definite time or has not yet been announced), 1 New Drug Marketing Application (NDA) approval, and 1 NDA acceptance. In March this year, Keji Pharmaceutical's Zewokiolenza injection was approved for marketing for the treatment of adult patients with relapsed or refractory multiple myeloma. It has progressed after at least 3 lines of previous treatment (at least one proteasome inhibitor and immunomodulator). In June,"Amimetoxin Injection" independently developed by Platinum Excellence became the first product in the country to receive NMPA new drug marketing application and be included in priority review. The indication is acute graft-versus-host disease (aGVHD) with hormone failure.).
Among the candidate products with announced targets, it can be seen that CD19 and BCMA are still popular targets, but there are also emerging targets such as LILRB4, CD99, and KRAS G12D, which may bring new options to disease treatment in the future. From the perspective of indications, stem cell candidate products target a wide range of indications. Other cell therapies are basically concentrated in the field of tumors, and a few immune cell therapies have entered the field of self-immune diseases.
According to past data, 36 cell therapies have made clinical application progress in Q1 in 2023, including 21 immune cell therapies and 15 stem cell therapies; 20 cell therapies have made clinical progress in Q2, including 16 immune cell therapies and 4 stem cell therapies. Overall, this year's H1 progress is not much different from last year's. It belongs to "progress while maintaining stability" and still maintains a strong development momentum.
Stem cell therapy
In the stem cell field, there are 22 candidate products that have applied for and/or approved INDs, accounting for nearly 40% of the total, occupying an important position. As Platinum Biotech's "Amimetoxin Injection" NDA has been accepted and included in the priority review, the domestic stem cell field is expected to usher in the first marketed product to fill the gap in the near future.
Among the candidate products submitted and accepted by the IND, these drugs cover various types such as human umbilical cord mesenchymal stem cells, hematopoietic stem cells, uterine blood mesenchymal stem cells, and fat mesenchymal stem cells. Mainly, beta-thalassemia, stroke, lung disease, etc. are indications with many stem cell therapies.
Among them, Quansheng Biotech's "Human Umbilical Cord Mesenchymal Stem Cell Injection" was approved for clinical trials in February this year for use in chronic obstructive pulmonary disease. This is also the fifth stem cell clinical trial approved by Quansheng Biotech by CDE. project. In May, Quansheng Biotech submitted another IND for "Human Umbilical Cord Mesenchymal Stem Cell Injection" and is expected to win another clinical project.
CAR-T therapy
In the CAR-T field, a total of 16 product candidate applications and/or approvals for INDs and approval for marketing. Reindeer Bio is actively expanding the indications of its marketed CAR-T therapies. In addition to hematological tumors, its IND for myasthenia gravis has also been approved for clinical use in January. At the same time, RD118, which targets GPRC5D, has also been approved for clinical use and has made great progress in the first half of 2024.
Companies such as Keji Pharmaceutical, Yimiao Shenzhou, Tuoxin Tiancheng, Yi Mufeng, Boruida Biotech, Sabiman Biotech, Minghui Gene, and Youkadi have all chosen to avoid the popular CD19 and BCMA and explore alternative ways. Keyi Pharmaceutical's BCMA/CD19 dual-target CAR-T product KQ-2003 has been approved for clinical tacit approval for POEMS syndrome. This is also the world's first CAR-T cell therapy used to treat POEMS syndrome.
Yizun Biotech has developed a CNK-UT002, which uses self-developed CNK-UT technology. This is a universal cell technology that integrates the two major platform technologies of CNK-T and U-T. T cells are collected from healthy donors and subjected to gene editing. After modification, it gives NK cells recognition function and has the activation and attack ability of T cells, which can specifically identify and treat tumors.
TIL/TCR-T/other T cell types
The clinical registration progress of TIL therapies in the first half of the year was mainly Baiji Biotech's BST02 and Junsai Biotech's GC203. Both of these have been approved for clinical use. The world's first TIL therapy has been approved for marketing, and the domestic TIL therapy track has also gradually accelerated.
In the field of TCR-T, a total of 4 TCR-T therapies were submitted for IND/approved clinical trials in the first half of 2024. TCR-T has diversified targets and focuses on solid tumors. There are also more than 10 companies on the domestic TCR-T track. Layout, most of them have entered the clinical research stage, and the fastest one has been advanced to Phase 2 clinical trials. It is worth mentioning that the world's first TCR-T therapy is also about to be approved and is expected to achieve a major breakthrough.
In addition, domestic companies are also continuing to make efforts in innovation, such as Ruishun Biotech's CAR-DNT therapy RJMty19, Huaxia Yingtai's STAR-T therapy YTS104, and Ji Dekangmin's γδ-T therapy. These pharmaceutical companies are all seeking more effective and safer therapies.
NK/CAR-NK/iNKT cell therapy
In the field of NK cells, a total of 6 candidate products have made progress, including Xianbo Biotech, Jingshan Biotech, Inbury, Enkaisai Pharmaceutical, Maideton Pharmaceutical, and Genome Qiming. Indications for NK cell therapy include hematological tumors, solid tumors and autoimmune diseases.
It is worth mentioning that Geni Qiming's GKL-006 injection is an iNKT cell therapy, which is the only one that can activate NK cells of the innate immune system and helper T cells of the acquired system through the adjuvant effect of IFN-γ., killer T cells and γδ-T cells. In addition, Inbury's IBR822 is the world's first non-viral, non-genetically modified NK cell (CAR-raNK) product that can specifically target TROP2 antigen and have the ability to kill tumor cells. It has demonstrated strong pre-clinical anti-tumor ability.
The NK cell therapy track is booming, and many types have entered clinical practice last year and since this year, initially demonstrating the potential of this therapy and is expected to continue to be exerted in the future.
Summary
Overall, cell therapy has maintained a steady growth trend in recent years, indications are gradually expanding, and stem cells are increasingly becoming an important main force for many unmet clinical needs. From the Timeline, we can see that many subdivided cell therapy tracks have made important breakthroughs, including the successful achievement of the "first" innovation from pre-clinical to clinical, the release of outstanding clinical data, etc....
Looking to the future, the following trends will be shown in the field of cell therapy: First, policy support will continue to strengthen; second, technological innovation will continue to accelerate; third, market competition will become more intense; fourth, clinical applications will become more extensive. As these trends continue to develop, we have reason to believe that cell therapy will play a more important role in the future medical field, bringing new treatment options to more patients.