
At present, 11 products have been approved for marketing on the CAR-T Track around the world, and TIL and TCR-T Track have also welcomed the first products on the market. Cell therapy is experiencing unprecedented rapid development. According to a nova one advisor research report, the global cell therapy market size will be US$4.85 billion in 2023 and is expected to exceed US$37.42 billion by 2033. The compound annual growth rate will be 22.67% in the next decade, and the market size will steadily expand.

▲ 2023-2033全球细胞疗法市场规模(图片来源:参考资料1)
In this context, in order to further improve treatment accessibility and reduce costs, the development of universal cell therapies around the world is showing strong momentum. Gamida Cell's Omisirge was approved by the FDA in April last year, becoming the first off-the-shelf stem cell transplant therapy approved, laying a solid foundation for the commercialization of universal cell therapies.
It is particularly worth mentioning that in the exploration of universal cell therapies, iPSC-derived cell therapies have become one of the mainstream development directions in this field due to their unique advantages. Among them, nearly 35 domestic companies have deployed the iPSC field, and as the global cell therapy market continues to expand, universal models, as an important direction of cell therapy, are expected to be commercialized in the future with their advantages of high accessibility. Demonstrate huge potential and occupy a certain share of the market.
Innovation and compliance go hand in hand, and GMP-level cytokines unlock differentiation potential
Whether it is universal immune cell therapy or iPSC regenerative therapy, the common key link in research and development lies in cell differentiation, which can naturally differentiate into different cell types under appropriate culture conditions, or can be induced by applying physical or chemical signals. Differentiation, and in this process, cytokines play an important role.
There are many types of cytokines, including growth factors (GF), interleukin (IL), interferon (IFN), colony stimulating factor (CSF), tumor necrosis factor (TNF), transforming growth factor-beta family, chemokines, etc. Different cytokines play different regulatory roles on cell growth and differentiation processes. Some cytokines can induce immature or immature cells to differentiate in specific directions to form cell types with specific functions, while others may play a role in promoting proliferation, regulating gene expression, and maintaining differentiation.
Among them, IL is a group of cytokines produced by a variety of cells, of which at least 38 have been discovered. IL-2, IL-7, IL-15 and IL-21 all play important roles in cell differentiation and proliferation. Taking the preparation of CAR-T cell therapy as an example, IL-2 is often used for T cell expansion in vitro, which can promote the expansion of cytotoxic T lymphocytes and the development of terminally differentiated effector T cells;IL-7 can selectively stimulate CAR-T cell proliferation while improving the anti-tumor activity of CAR-T cells;IL-15 can not only enhance the anti-tumor activity of CAR-T cells, but can affect the cell phenotype and improve persistence;IL-21 can promote the maturation of CD8 +T cells and enhance their cytotoxicity.
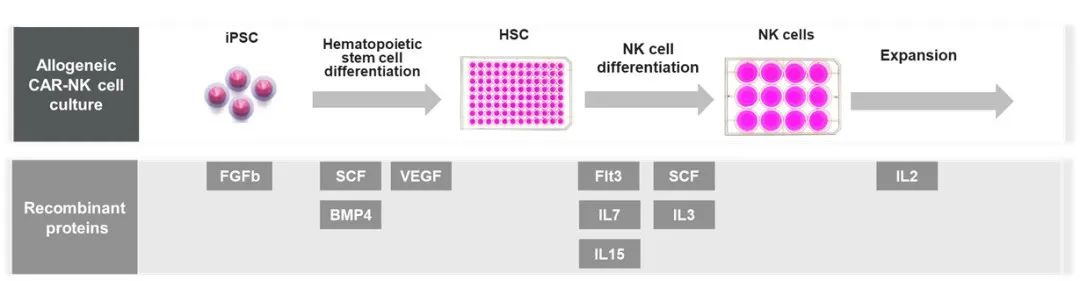
▲ Preparation process of iPSC CAR-NK and the cytokines involved
In the preparation process of iPSC-derived CAR-NK cell therapy, the main links involved include the acquisition and reprogramming of iPSC, the targeted differentiation of iPSC into hematopoietic stem cells (HSC), and then differentiation into NK cells, the introduction of CAR, and CAR-NK expansion and purification, etc. Many aspects such as directional differentiation and amplification require the participation of exogenous cytokines in regulation, thereby improving differentiation efficiency and increasing the purity of the final product. At present, cytokines have been widely used in various cell differentiation processes. With the gradual rise of universal cell therapies, the demand for them is also increasing.
In April 2023, the "Technical Guidelines for Pharmaceutical Research and Evaluation of Human Stem Cell Products (Trial)" issued by the Center for Drug Evaluation of the State Food and Drug Administration mentioned that "if research-grade reagents (such as culture medium, cytokines, chemical small molecules, etc.) are used in production, in order to ensure product consistency and purity, production should be carried out in accordance with GMP requirements as much as possible." However, there is currently no broad consensus on the standards for cytokine products, and there are differences between different products. With the increasing popularity of market research and development, companies pursuing better efficacy and safety of candidate products, and increasingly stringent supervision, researchers 'selection criteria for cytokine products are also increasing day by day. There is an urgent need to stabilize large-scale supply and Cytokines that can meet GMP standards to meet the needs of research and development and subsequent registration.