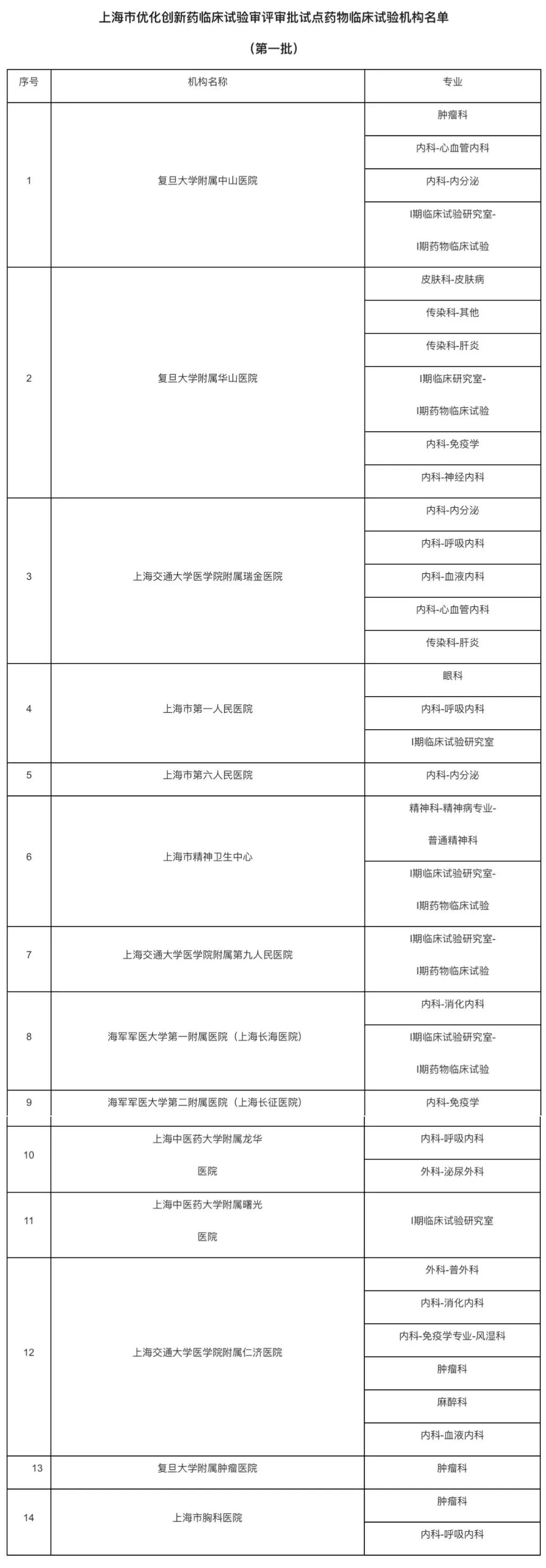
Source: Shanghai City Food and Drug Administration
According to the Shanghai City Food and Drug Administration, according to the "Notice of the State Food and Drug Administration on Printing and Issuing the Pilot Work Plan for Optimizing the Review and Approval of Clinical Trials of Innovative Drugs"(State Food and Drug Administration Note [2024] No. 21) and the "Notice of the Shanghai City Drug Administration on Printing and Issuing the Work Plan for Optimizing the Review and Approval of Clinical Trials of Innovative Drugs"(Shanghai Food and Drug Administration Note [2024] No. 202) Relevant requirements, after review and evaluation of the application materials, 14 drug clinical trial institutions (37 majors) in this city were determined to be the first batch of pilot drug clinical trial institutions for optimizing the review and approval of innovative drugs clinical trials. See the annex ↓