
MSCs can be administered by injection or surgery (i.e., transplanted). Intraarticular injections are most commonly used because it is a relatively simple and safe procedure and can also be used for outpatient care. However, this technique does not ensure the correct injection of cells in the target area. In contrast, surgical implantation of MSCs is more invasive, but can overcome this limitation and ensure accurate cell deposition in the target area. Many cell therapies for knee OA are easy to implement due to their autologous nature and minimal procedures required. It is worth noting that the use of MSC has always been proven to be safe, while they will not hinder additional treatments in the future if treatment fails. These treatments appear to be effective in reducing pain and improving function, but little is known about their impact on cartilage regeneration and disease improvement in clinical practice.
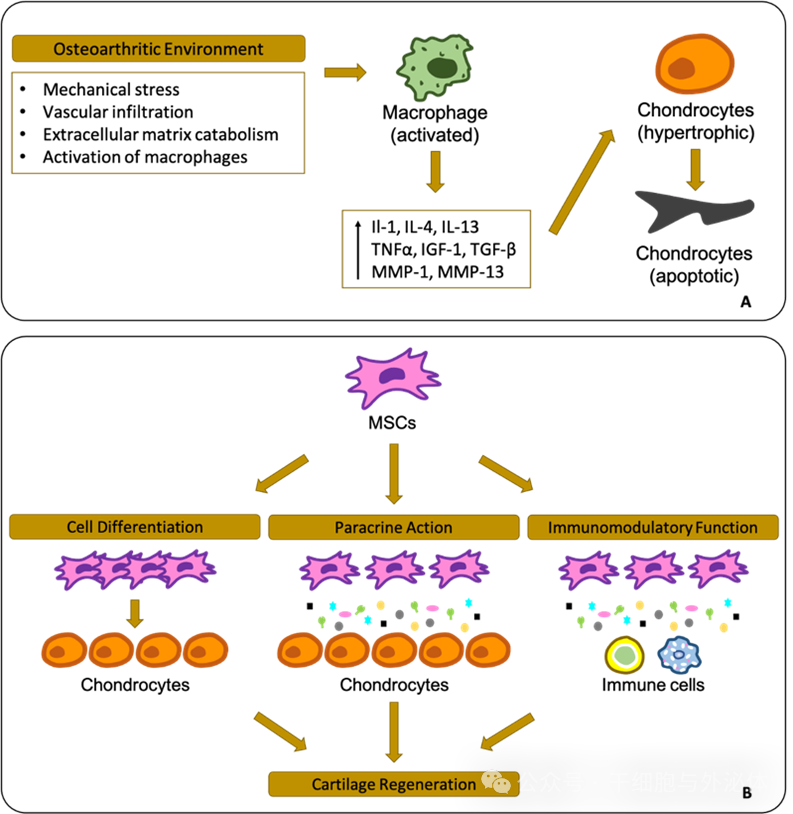
Figure: Schematic diagram of the osteoarthritis environment and cellular reactions in the joint (A). Based on preclinical data, mesenchymal stem cells (MSC) have a high regenerative capacity (B).
1. Common doses of MSCs injection for knee joint treatment
The most common way to administer MSCs is intraarticular injection. Most clinical protocols recommend that intraarticular injections of 10 to 40 x 10^6 MSCs per time tend to show better results. Current literature shows that intra-articular injection of MSCs has achieved encouraging results in reducing pain, improving functional outcomes and overall quality of life. Initially, most relevant articles were non-randomized studies or case series. However, recent studies have demonstrated that MSCs provide better efficacy at 6 and 12 months compared to placebo and hyaluronic acid injections. A single intra-articular injection of MSCs in patients with knee OA was associated with improvement of symptoms. Overall, 94.1% of the included studies reported that MSCs or derived products were more effective than the control group. Today, there are two main sources of MSC implantation: autologous adipose tissue-derived mesenchymal stem cells (AD-MSC) or umbilical cord allogeneic (hUCB-MSC). Prior to implantation, adipose tissue is collected from the patient's abdominal or hip area by simple liposuction. On the other hand, hUCB-MSCs are obtained at delivery from maternal umbilical veins and arteries or placental tissue. As more cells are used, cultural expansion of these two sources may enhance their effects. MSC is usually embedded in or mixed with three-dimensional scaffold materials, including hyaluronic acid, collagen, or fibrin glue.
2. Mesenchymal stem cells treated 467 cases of bones and joints, with encouraging functional results
Recent studies have shown that good results can be achieved using patient-reported outcome measurements (PROM), radiographic assessments, or secondary arthroscopy.

Researchers in South Korea published a clinical observation report titled Mesenchymal Stem Cell Implantation in Knee Osteoarthritis: Midterm Outcomes and Survival Analysis in 467 Patients in the industry journal Orthop J Sports Med.
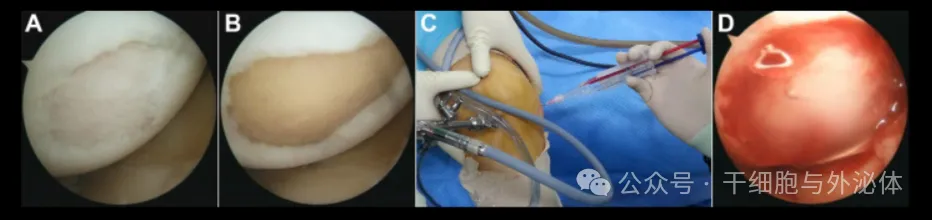
Figure: Arthroscopic implantation of fibrin glue loaded mesenchymal stem cells. (A)Damage to the articular cartilage on the medial femoral condyle was noted. (B)Precise debridement of all unstable and damaged cartilage at the injury site. (C)A cell-thrombin-fibrinogen suspension was applied to the lesion. (D)After manipulation with the probe, the cartilage lesions were covered with a cell-thrombin-fibrinogen suspension. Scientists Kim et al. conducted a large case series to assess mid-term clinical outcomes and survival in 467 patients with knee OA who received AD-MSC implantation on a fibrin glue stent and were followed up for at least 5 years.
The study showed encouraging functional results, acceptable duration of symptom relief, and 5-and 9-year survival rates of 99.8% and 74.5% respectively in terms of conversion to high tibial osteotomy or knee arthroplasty. 3. Mesenchymal stem cells treat 128 cases of bones and joints, significantly improving pain and function. In another study, South Korean scientist Song et al. published a series of large cases, including 128 patients with Kellgren-Lawrence (KL) grade 1 to 3 knee osteoarthritis who received hUCB-MSCs implantation combined with hyaluronic acid (HA) hydrogel treatment and were followed up for at least two years (picture below).
 文Presentation:Implantation of allogenic umbilical cord blood-derived mesenchymal stem cells improves knee osteoarthritis outcomes: Two-year follow-up
文Presentation:Implantation of allogenic umbilical cord blood-derived mesenchymal stem cells improves knee osteoarthritis outcomes: Two-year follow-up
The researchers concluded that implantation of UCB-MSC-HA significantly improved pain and function without adverse reactions or postoperative complications. Radiological assessments using the modified MOCART score were also performed 3-6 months and one year after surgery and showed increased values (30.58 on the first MRI and 55.44 on the second MRI). Compared with preoperative scores, the mean (standard deviation)VAS, WOMAC, and IKDC scores (including hUCB-MSC implantation) improved significantly at 1 and 2 years postoperatively.
4. Mesenchymal stem cells treated 176 cases of bones and joints, and better cartilage healing
It should be noted that a key point in treating knee osteoarthritis is prioritizing treatment. Limb alignment should be evaluated first, then joint stability should be evaluated, and then meniscus and cartilage surgery should be considered next. In this regard, when there is significant varus, MSC administration is often combined with high tibial osteotomy (HTO).

In fact, a study conducted by researcher Ren Jiong at the Affiliated Hospital of Jeonnam National University School of Medicine in South Korea demonstrated the effectiveness of this combined surgery (above). That is, 176 patients who underwent high tibial osteotomy (HTO) combined with mesenchymal stem cell (hUCB-MSC) surgery for medial compartment osteoarthritis were followed for at least 2 years. Clinical results were evaluated using different PROMs (IKDC, KOOS, SF-36, Tegner) and the results showed significant improvements in both groups, with no differences between the two groups. However, a second arthroscopy showed better cartilage healing in the hUCB-MSC group.
In summary, MSCs are increasingly being used to treat knee OA, whether as intra-articular injections (most common) or surgically implanted into the lesion site with stents. They are very effective in alleviating pain, improving function and quality of life.