
The research and development of new stem cell drugs in China is advancing at a new speed. Nearly 103 clinical trial applications for stem cell drugs have been accepted, and many of these new stem cell drugs have entered the clinical research stage.
In recent years, driven by policies, my country's stem cell industry has expanded rapidly, and the research and development of new stem cell drugs has accelerated significantly. According to statistics, as of September 2, 2024, a total of 103 domestic applications for clinical trials of stem cell drugs have been accepted.
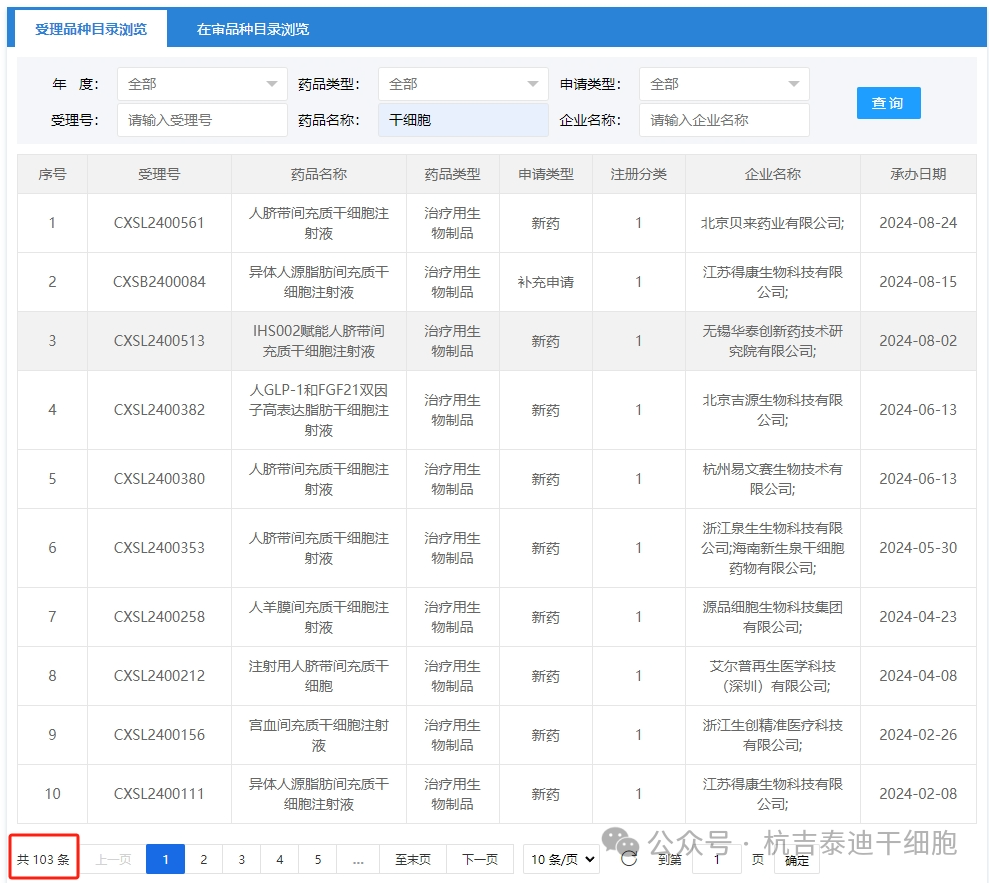
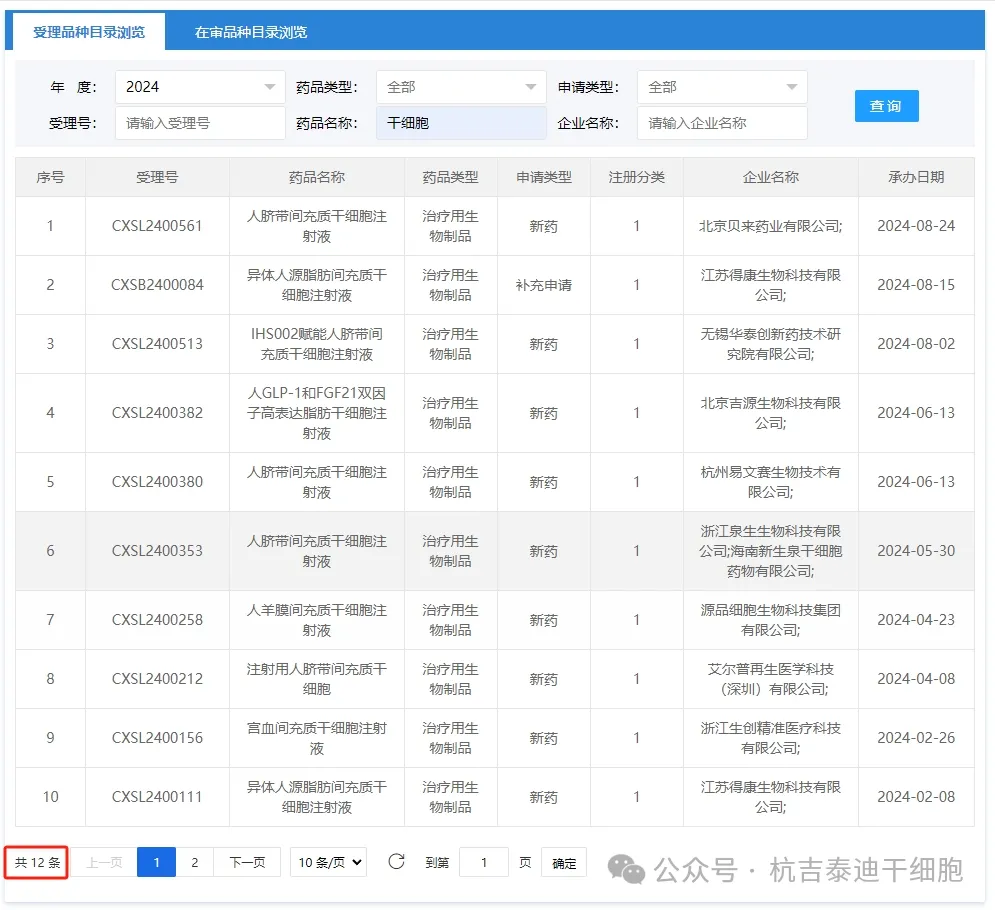
Among them, as of January 1 to September 2, 2024, the number of new applications accepted has reached 12.
Among them, as of September 1, 2024, a total of 110 items from 54 companies (excluding subsidiaries) have been allowed to acquiesce in entering clinical trials (implied approval for clinical trials).
These new stem cell drugs cover many types, such as mesenchymal stem cells, hematopoietic stem cells, neural stem cells, etc., and are widely used to treat nervous system diseases, cardiovascular and cerebrovascular diseases, and blood system diseases. At present, many stem cell drugs have entered clinical phase III, indicating that China's new stem cell drug research and development is about to usher in explosive growth.
As of September 2, 2024, 12 new stem cell drugs have been accepted
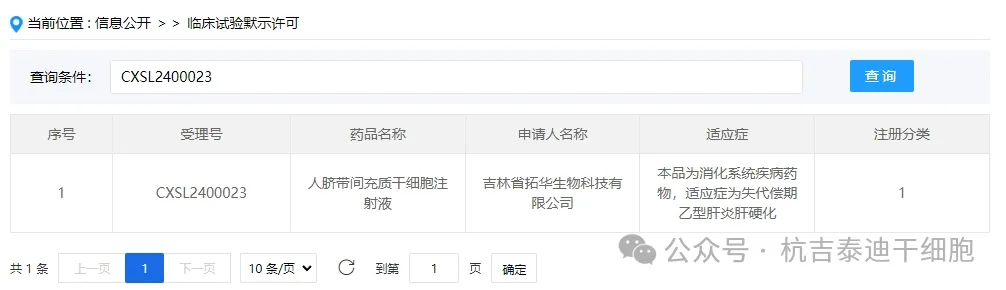
Item 1 of the acceptance of new stem cell drugs in 2024: On January 10, 2024, the clinical trial application of "Human Umbilical Cord Mesenchymal Stem Cell Injection" from Tuohua Biotechnology Co., Ltd. of Jilin Province was accepted (acceptance number: CXSL2400023).
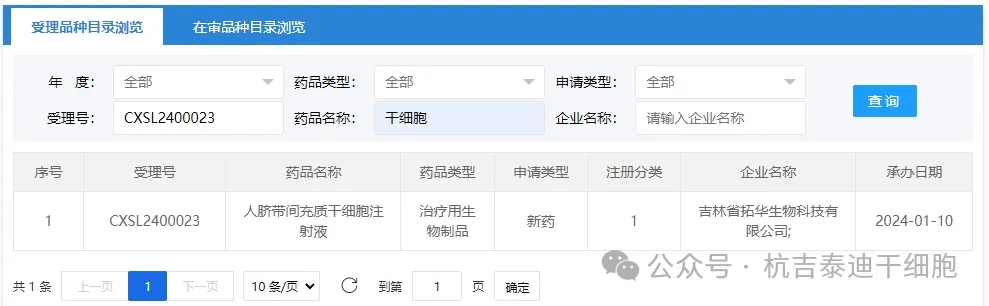
The indication for this new stem cell drug is for digestive system diseases, and the indication is decompensated hepatitis B cirrhosis.
This is the second indication for this cell injection that has been accepted. As early as February 23, 2023, Suzhou Tuohua Biotechnology Co., Ltd.'s "Human Umbilical Cord Mesenchymal Stem Cell Injection" has been obtained with tacit approval (Acceptance No.: CXSL2300152). The indication for this new stem cell drug is moderate to severe acute respiratory distress syndrome.
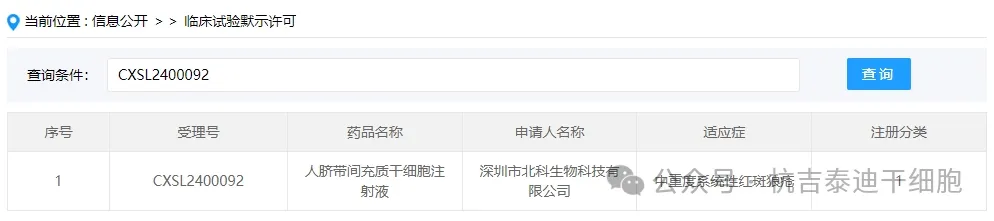
Item 2 of new stem cell drug acceptance in 2024: On February 10, 2024, Shenzhen Beike Biotechnology Co., Ltd.'s clinical trial application for "Human Umbilical Cord Mesenchymal Stem Cell Injection" was accepted (acceptance number: CXSL2400092).
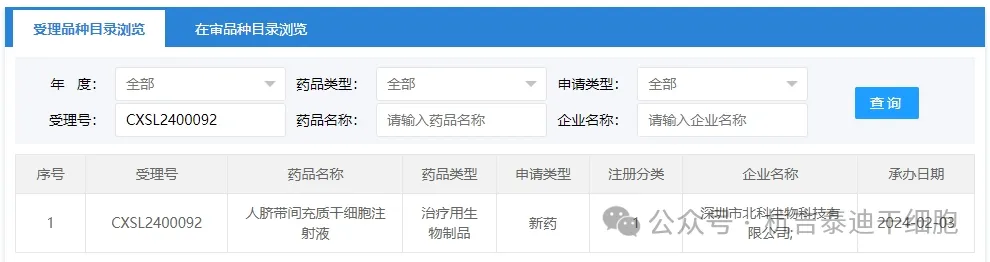
The indication for this new stem cell drug is moderate to severe systemic lupus erythematosus. Previously, this stem cell preparation was accepted on June 5, 2020 (acceptance number: CXSL2000128), but the progress of subsequent tasks cannot be inquired.
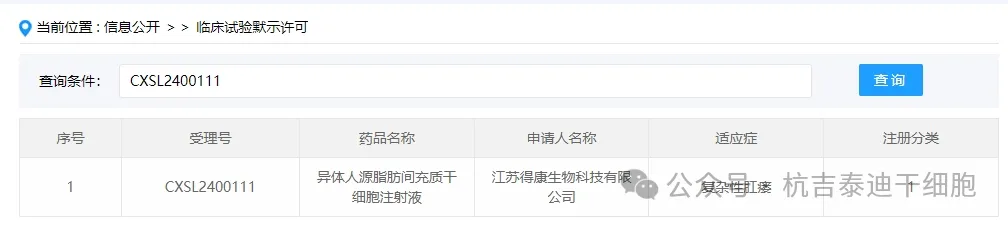
Item 3 of the acceptance of new stem cell drugs in 2024: On February 08, 2024, Jiangsu Dekang Biotechnology Co., Ltd.'s clinical trial application for "Allogeneic Human Fat Mesenchymal Stem Cell Injection" was accepted (acceptance number: CXSL2400111).
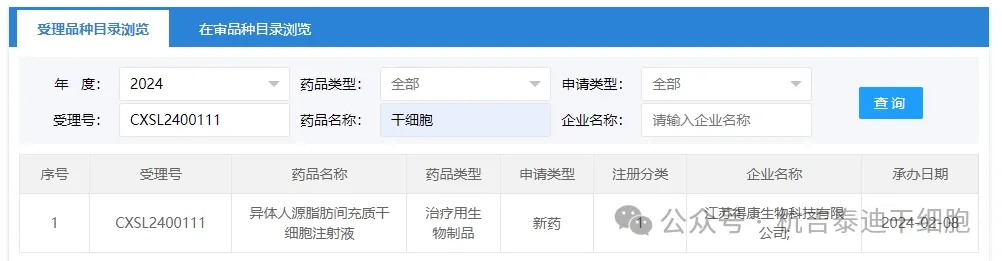
The indication for this new stem cell drug is complex anal fistula.
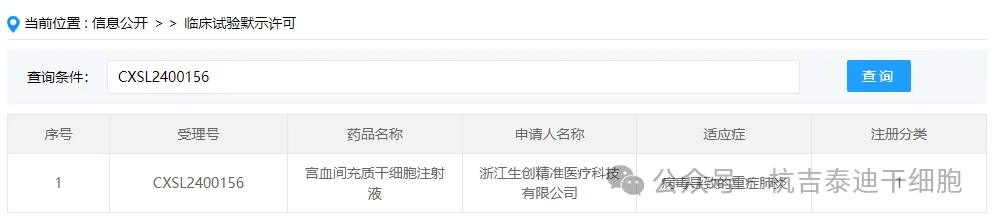
Item 4 of the acceptance of new stem cell drugs in 2024: On February 26, 2024, Zhejiang Shengchuang Precision Medical Technology Co., Ltd.'s clinical trial application for "Gongxue Mesenchymal Stem Cell Injection" was accepted (acceptance number: CXSL2400156).
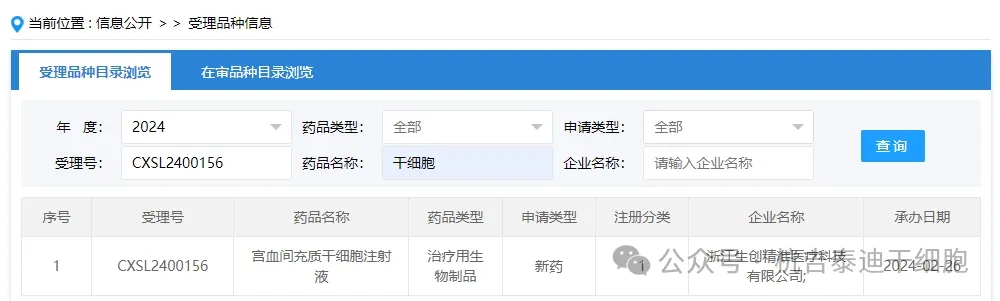
The indication for the company's new stem cell drug is severe pneumonia caused by the virus.
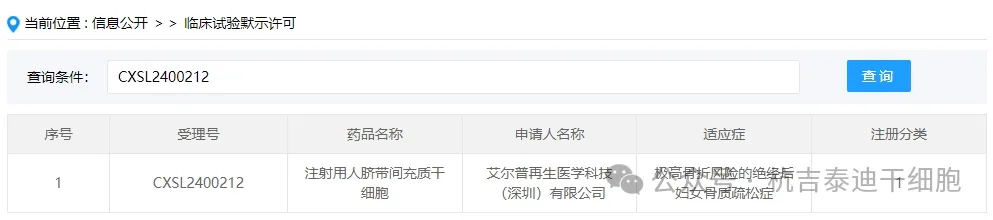
Item 5 of the acceptance of new stem cell drugs in 2024: On April 08, 2024, Aierp Regenerative Medicine Technology (Shenzhen) Co., Ltd.'s clinical trial application for "Human Umbilical Cord Mesenchymal Stem Cells for Injection" was accepted (acceptance number: CXSL2400212).
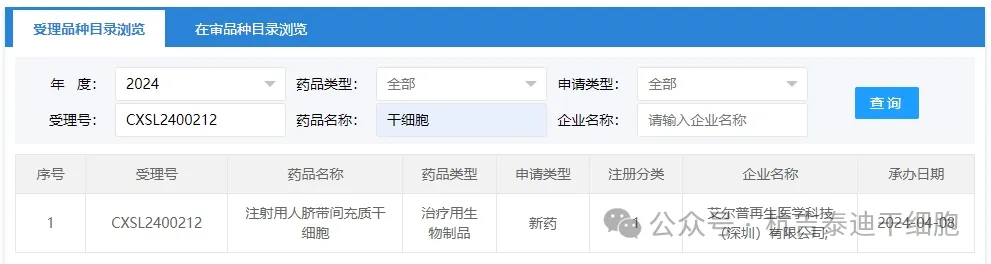
The company's new stem cell drug is indicated for osteoporosis in postmenopausal women who are at extremely high risk of fractures.
Item 6 of the acceptance of new stem cell drugs in 2024: On April 23, 2024, Yuanpin Cell Biotechnology Group Co., Ltd.'s clinical trial application for "Human Amnion Mesenchymal Stem Cell Injection" was accepted (acceptance number: CXSL2400258).
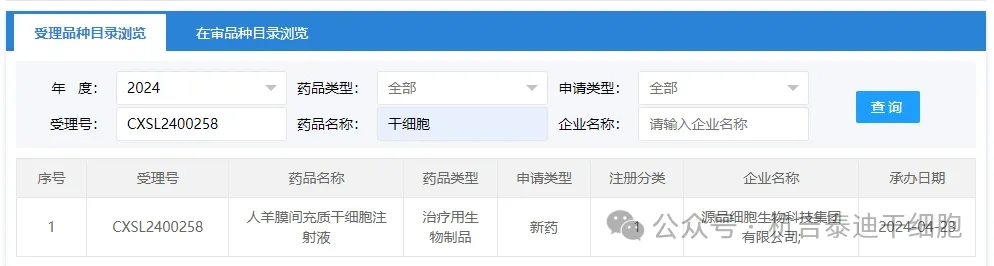
The indications for the company's new stem cell drug have not been inquired yet.
Item 7 of the acceptance of new stem cell drugs in 2024: On May 30, 2024, Zhejiang Quansheng Biotechnology Co., Ltd.; Hainan Xinshengquan Stem Cell Drug Co., Ltd.'s clinical trial application for "Human Umbilical Cord Mesenchymal Stem Cell Injection" was accepted (Acceptance No.: CXSL2400353). This is the sixth time that this cell injection has been accepted.
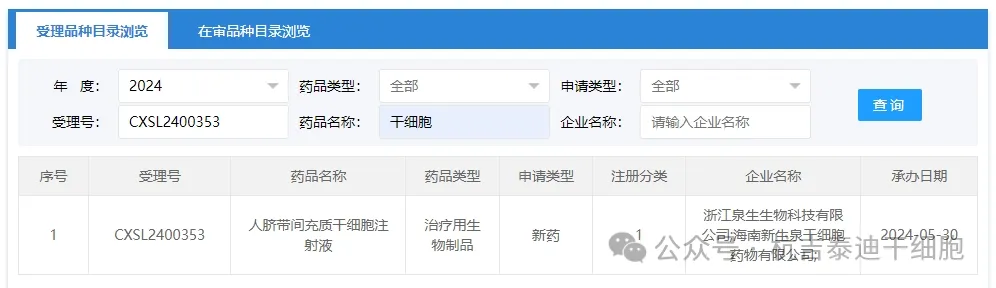
The indication for the new stem cell drug accepted by the company is: previously implicitly approved for injecting drugs for the treatment of patients with mild to moderate acute respiratory distress syndrome (ARDS)(Acceptance No.: CXSL2200114), injection for ankylosing spondylitis (Acceptance No.: CXSL2200299), injection for second-degree burns (Acceptance No.: CXSL2200301), injection for decompensated hepatitis B virus cirrhosis (Acceptance No.: CXSL2200457), and injection for chronic obstructive pulmonary disease (Acceptance No.: CXSL2300795).
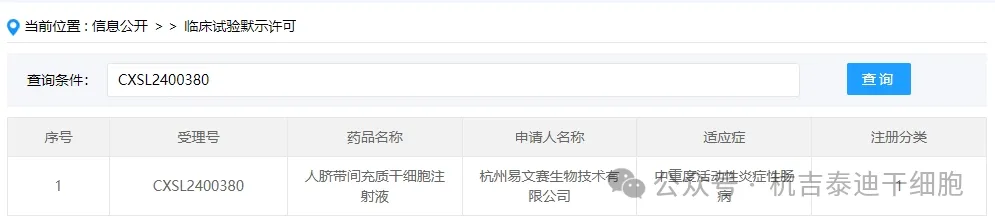
Item 8 of the acceptance of new stem cell drugs in 2024: On June 13, 2024, Hangzhou Yiwensai Biotechnology Co., Ltd.'s clinical trial application for "Human Umbilical Cord Mesenchymal Stem Cell Injection" was accepted (acceptance number: CXSL2400380) was accepted.
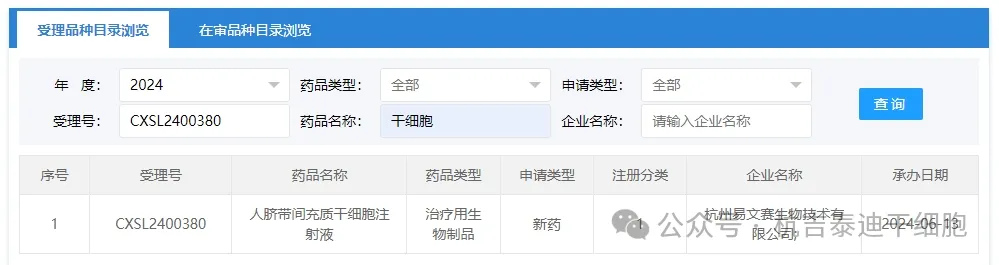
The indication for the company's new stem cell drug is moderately to severely active inflammatory bowel disease.
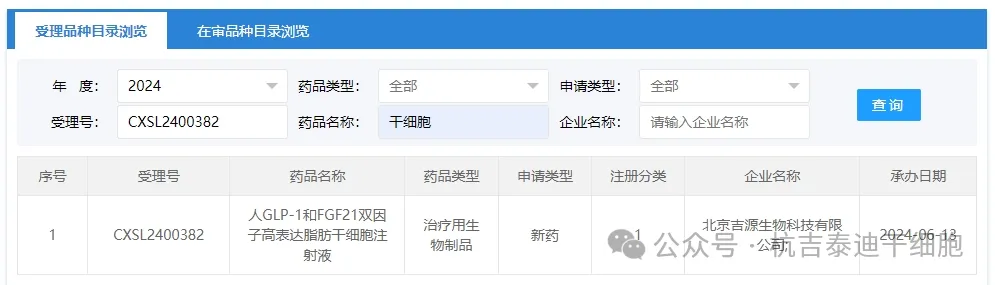
Item 9 of the acceptance of new stem cell drugs in 2024: On June 13, 2024, Beijing Jiyuan Biotechnology Co., Ltd.'s clinical trial application for "Human GLP-1 and FGF21 Dual Factor High-Expression Adipose Stem Cell Injection" was accepted (acceptance number: CXSL2400382).
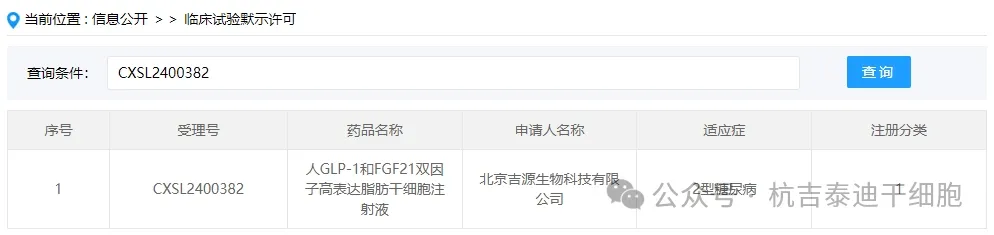
The indication for the company's new stem cell drug is type 2 diabetes.
Item 10 of new stem cell drugs acceptance in 2024: On August 02, 2024, Wuxi Huatai Innovative Pharmaceutical Technology Research Institute Co., Ltd.'s clinical trial application for "IHS002 Empowering Human Umbilical Cord Mesenchymal Stem Cell Injection" was accepted (acceptance number: CXSL2400513).
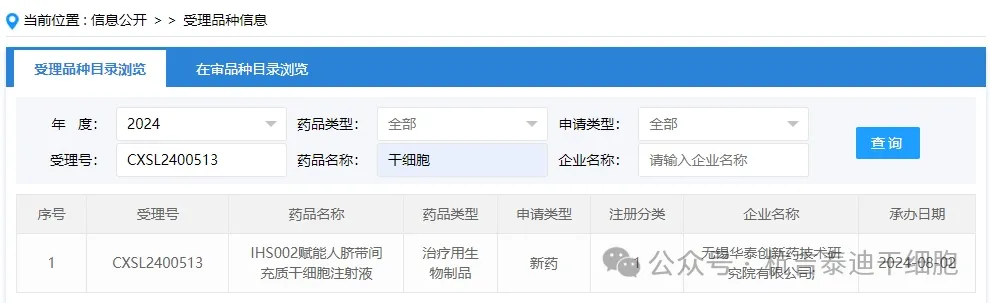
The indications for the company's new stem cell drug have not been inquired yet.
Item 11 of the new stem cell drug acceptance in 2024: On August 15, 2024, Jiangsu Dekang Biotechnology Co., Ltd.'s clinical trial application for "Allogeneic Human Fat Mesenchymal Stem Cell Injection" was accepted (acceptance number: CXSB2400084) was accepted.
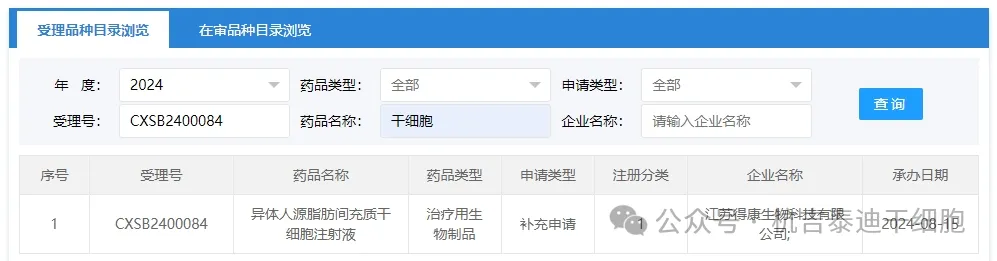
The indications for the company's new stem cell drug have not been inquired yet.
Item 12 of the new stem cell drug acceptance in 2024: On August 24, 2024, Beijing Beilai Pharmaceutical Co., Ltd.'s clinical trial application for "Allogeneic Human Umbilical Cord Mesenchymal Stem Cell Injection" was accepted (acceptance number: CXSL2400561). This is the fifth time that this cell injection has been accepted.
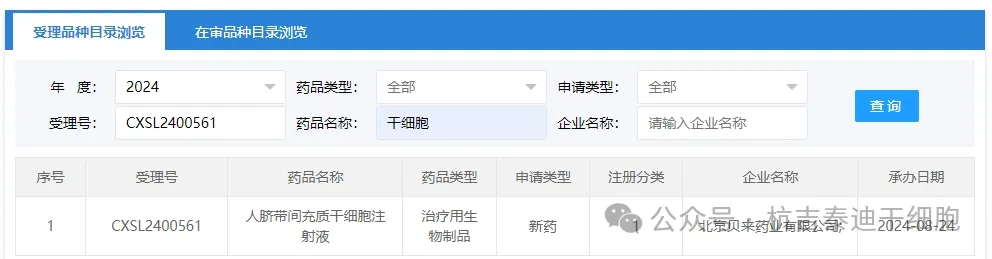
The indications for new stem cell drugs accepted by the company are: This is the fifth indication of this stem cell injection accepted,(acceptance number: CXSL2400561, indication unknown),(acceptance number: CXSL2300776, indication unknown), rheumatoid arthritis (CXSL2000005), idiopathic pulmonary fibrosis (CXSL2200370), Alzheimer's disease (CXSL2300129).
looking ahead
The global field of cell therapy has achieved many major original results in the field of basic research, and our country is among the best.
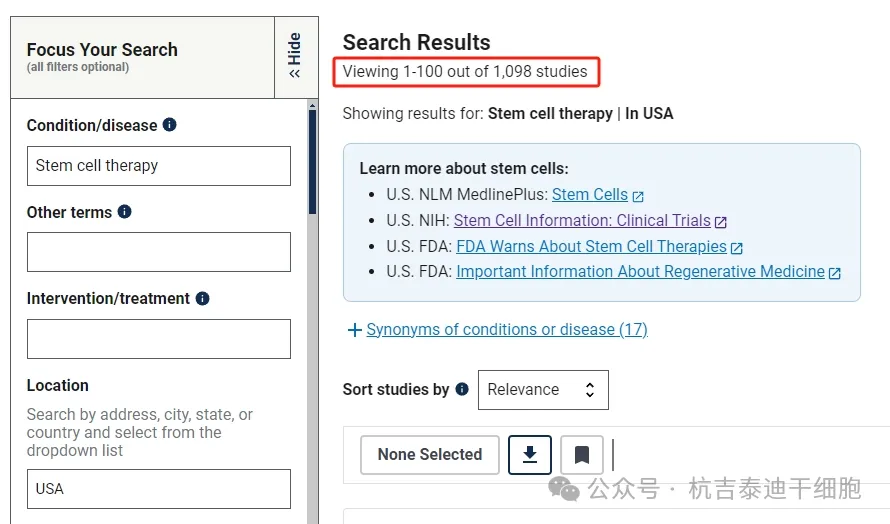
As of September 2024, there are 2693 clinical research projects related to stem cell therapy registered on the National Institutes of Health's largest clinical trial registry, clinicaltrials.gov.
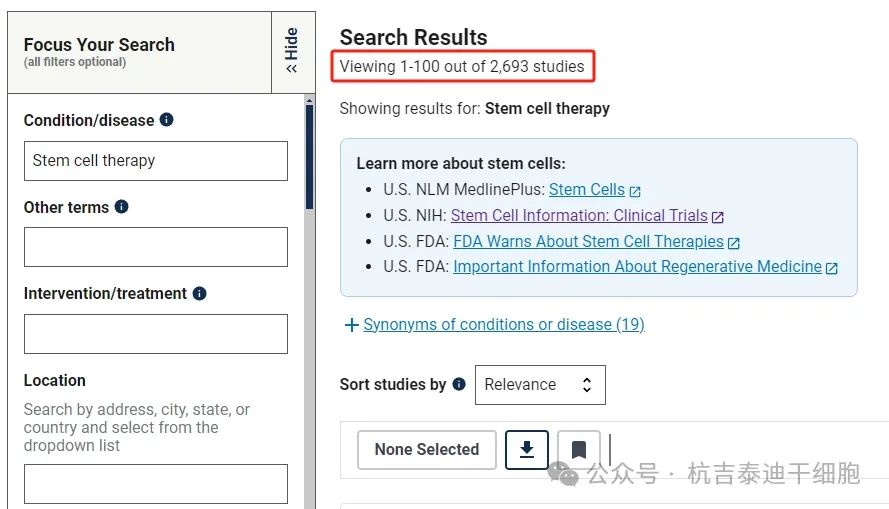
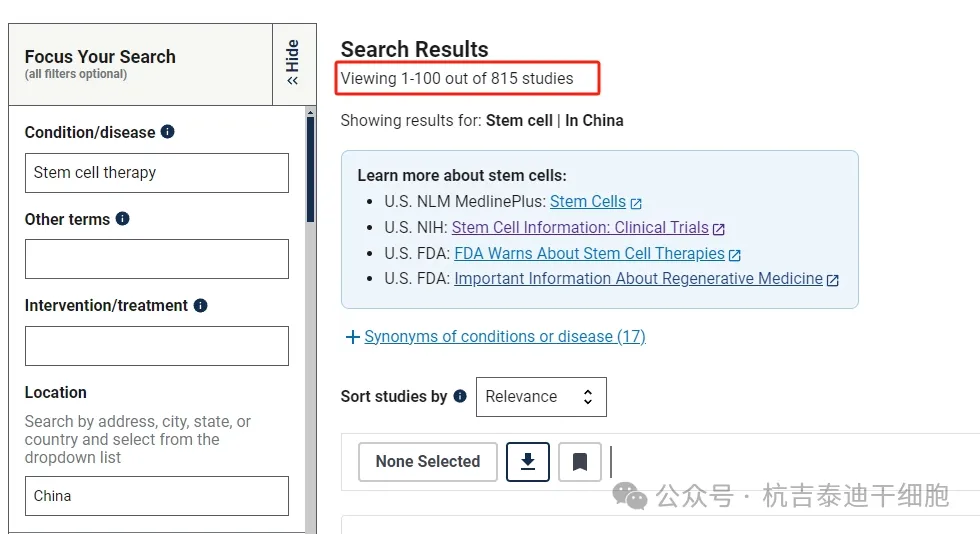
Among them, there are 815 stem cell clinical research projects conducted in China and 1098 in the United States. China and the United States are on par with each other in stem cell engineering technology and genetic engineering technology.
It is believed that 2024 will be an explosion period for stem cell drug research and development, and more cell drugs for new indications will enter the clinical trial stage. At the same time, the ongoing Phase I/II clinical trials of stem cell drugs will gradually produce results. It is believed that with the continuous deepening of research and development of stem cell technology, stem cells will be on the market as therapeutic drugs in the near future.