
Diabetes threatens the physical and mental health of about 537 million people around the world. There are 130 million people with diabetes in China, and the incidence rate is increasing year by year. It is expected that by 2030, the number of people with the disease will soar to 643 million! Given the limitations of existing treatment methods, researchers have set their sights on regenerative therapy in recent years.
It is gratifying to note that good news has come again in the field of diabetes recently, and China and foreign countries have made breakthroughs!
Type 1 and type 2 diabetes are very different
Diabetes mellitus (DM) is a severe and high-incidence chronic metabolic disease characterized by absolute or relative insulin deficiency or insulin resistance. Depending on the cause, diabetes can be divided into the following types:
01 Type 1 diabetes mellitus (T1DM)
Due to the autoimmune reaction, the human body's immune system attacks and destroys the insulin-producing beta cells in the pancreatic islets. Pancreatic beta cells are the only producers of insulin in the body. Once damaged or destroyed, it may lead to the disappearance of insulin secretion. Therefore, people with type 1 diabetes usually need daily insulin injections to replenish unproduced insulin. Type 1 diabetes is usually diagnosed in childhood or early adolescence and requires continuous monitoring to reduce the risk of long-term diabetes complications and hypoglycaemia.
▼ Comparison of normal pancreatic β cells and pancreatic β cells in patients with type 1 diabetes
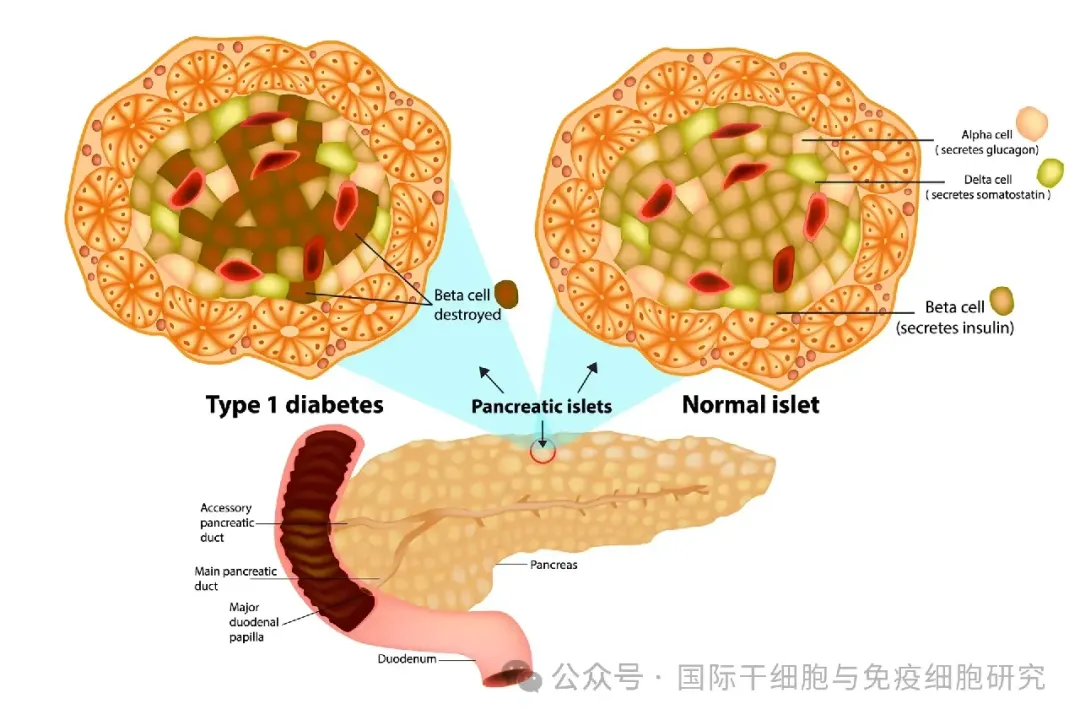
▲ Picture source "New Atlas"
02 Type 2 diabetes mellitus (T2DM)
Type 2 diabetes is mainly caused by insulin resistance and pancreatic beta cell secretion dysfunction, accounting for about 85% to 95% of all diabetic patients. About 30% of people with type 2 diabetes need insulin injections to control the condition.
03 other types of diabetes
Common such as gestational diabetes and special types of diabetes (in addition to the above three types, the general term for other diabetes).
China and foreign countries have recently updated two major breakthroughs in the field of diabetes cell therapy
01 Dawn of type 2 diabetes: China's first new class 1 genetically modified mesenchymal stem cell drug IND approved
At the end of August 2024, the Center for Drug Evaluation (NMPA) of the State Food and Drug Administration of China approved the new drug IND of "Human GLP-1 and FGF21 Dual Factor Highly Expressed Adipose Stem Cell Injection", which is intended to be used for the treatment of type 2 diabetes. This is China's first new class 1 genetically modified mesenchymal stem cell drug.
This therapy uses genetic modification technology to efficiently modify adipose stem cells so that they can express two important metabolic regulatory factors in patients 'bodies, namely glucagon-like peptide-1 (GLP-1, which inhibits glucagon release and promotes insulin secretion), fibroblast growth factor 21 (FGF-21, which has the dual effects of lowering blood sugar and lowering blood lipids), their ultimate goal is to regulate these two primers to improve islet function, regulate blood sugar, control weight, and ultimately achieve the goal of treating type 2 diabetes.
It is reported that this project has received strong support from the "National Key Research and Development Plan" of the Ministry of Science and Technology of China, marking an important step in China's field of genetically modified mesenchymal stem cell (MSC) drug research and development, bringing benefits to patients with type 2 diabetes. A new dawn!
02 New induced pluripotent stem cell islet replacement therapy (cell bag) helps type 1 diabetes patients without insulin for 4 years
▲ The screenshot comes from the "Sernova official website"
①Sernova cell bag: a new induced pluripotent stem cell islet replacement therapy
Sernova cell bag, a new type of induced pluripotent stem cell islet replacement therapy
The Sernova Cell Bag System is an implantable off-the-shelf induced pluripotent stem cell (iPSC) islet replacement therapy developed by Sernova and Evotec.
The treatment principle of this therapy is to use minimally invasive technology to implant this small implantable medical device under the skin of the patient's abdominal muscles. After implantation into the cell bag for about 6 weeks, the condition is stabilized by immunosuppressive therapy, and then the islet cells are transplanted into the vascularized tissue cavity formed by the cell bag. Because the device is porous, it will form a natural vascularized tissue environment in the body after implantation, allowing therapeutic cells to survive and function for a long time, releasing necessary factors that diabetic patients lack or lack, and is suitable for use in insulin-dependent diabetes.(Type 1 diabetes) treatment.
②2024 European Association for the Study of Diabetes: Phase I/II clinical study results are promising!
According to Sernova's official website, on September 12, 2024, the results of the first phase I/II clinical trial of Sernova cell bag transplantation (NCT03513939) were announced at the 2024 European Association for the Study of Diabetes (EASD) Annual Meeting. The study enrolled patients with type 1 diabetes aged 18 to 65 years with a history of more than 5 years and a history of severe hypoglycaemic episodes and hypoglycaemic unconsciousness, which means that these patients could not detect signs of dangerous hypoglycaemia. After enrollment, patients were divided into two groups: Group A (6 patients, receiving first-generation cell bag treatment) and Group B (7 patients, receiving cell bag treatment with a 50% increase in islet volume).
The results showed that all 6 patients in Group A were able to get rid of insulin treatment for a long time after receiving cell bag + donor islets combined transplantation, and glycosylated hemoglobin (HbA1c) was ≤6.5%(the normal value of HbA1c was approximately 5.7%~6.4%). Moreover, 5 years after cell bag transplantation, pathological examination confirmed that none of these patients showed signs of scar tissue or equipment degradation (such as material degradation or structural changes), and the tissue and blood supply were sufficient.
It is worth mentioning that the first patient to receive treatment had not been injected with insulin for more than 4 years and his blood sugar levels were in the non-diabetic range.
③People with type 1 diabetes take another step forward in getting rid of insulin dependence
It is reported that this study is the first evidence that islets maintain such a healthy level and function for such a long time in implantable and retrievable systems, and is a major advancement in cell therapy for the treatment of type 1 diabetes! Clinical trials in Group B are currently underway, let us wait and see the results.
In summary, the clinical research data of this world's first new induced pluripotent stem cell islet replacement therapy is of great significance. It brings practical hope for patients with type 1 diabetes to get rid of insulin dependence and takes it in the field of functional treatment of diabetes. An important step!
Summary
Diabetes is a metabolic disease that plagues the health of countless patients. Patients not only need long-term medication treatment, but the resulting complications, such as diabetic foot, diabetic nephropathy, etc., are also a serious threat to the patient's physical and mental health. With the continuous development of regenerative medicine such as stem cells, researchers are eager to find solutions at the cellular level.
Although stem cell treatment of diabetes is still in its early stages, more and more research at home and abroad in recent years has confirmed the effectiveness and safety of stem cell therapy in the treatment of diabetes, bringing new hope and choices to diabetic patients!