
Induced pluripotent stem cell (iPSC) derived mesenchymal stem cells (iMSC) provide a promising alternative to primary mesenchymal stem cells (MSC) and their derivatives, especially extracellular vesicles (EVs), that can be used in advanced therapeutic drugs.
New research demonstrates the great promise of iMSCs and iMSCs-EVs as alternative sources of MSC-derived therapies because of their powerful immunomodulatory and regenerative properties.
Mesenchymal stem cells (MSC) represent a population of stem cells that have good therapeutic effects in a variety of diseases involving tissue regeneration and the immune system.
As of September 30, 2024, a total of 98 items in my country have been approved to acquiesce in entering clinical trials (implicit approval for clinical trials). From January 1 to September 30, 2024, a total of 20 items were approved to acquiesce in clinical trials (implied approval for clinical trials), of which 3 items were added in the third quarter of 2024, all of which are derived from mesenchymal stem cells. Approved indications: decompensated liver cirrhosis, diabetic foot ulcer, ischemic stroke, moderate to severe systemic lupus erythematosus, etc.
However, complexities related to donor diversity, different tissue sources, and the need for extensive in vitro expansion continue to limit the use of MSC as an advanced therapeutic. To overcome these limitations, different, available sources of MSC must be identified. The generation of induced pluripotent stem cell (iPSC)-derived MSCs (iMSC) appears to provide an opportunity to effectively address most of these limitations. As an alternative source of pluripotent stem cells, iPSC can be produced from patient-specific adult somatic cells such as skin fibroblasts, peripheral blood cells, or other tissues through transcription factor-induced reprogramming. In addition, iPSC is considered an inexhaustible source of iMSC that can meet high clinical needs.
The research team from the United Kingdom and Australia published in the journal Nature Nat Med. The research results titled Production, safety and efficacy of iPSC-derived mesenchymal stromal cells in acute steroid-resistant graft versus host disease: a phase I, multicenter, open-label, dose-evacuation study proved that an iPSC line can produce 29 million clinical doses of iMSC for clinical treatment. In addition, iMSCs induced from individual iPSC cells or clones are theoretically more homogeneous.
In June 2024, the above-mentioned team published the research results titled Two-year safety outcomes of iPS cell-derived mesenchymal cells in acute steroid-resistant graft-versus-host disease in the sub-issue of the journal Nature, Nat Med. The first completed clinical trial of iPS cell-derived mesenchymal stem cells (iMSC) was conducted in 15 participants with steroid-resistant acute graft-versus-host disease. Safety, tolerability, and effectiveness were reported during the 100-day evaluation period after intravenous infusion of iMSC, and it was concluded that systemic infusion of iMSC was safe and well tolerated at 2-year follow-up and lasted up to 2 years after the first infusion. ClinicalTrials.gov Registration:NCT02923375.
Importantly, as a potentially unlimited source of MSCs, iMSCs are very similar to their primary tissue-derived MSCs in terms of morphology, immunophenotype, and trilineage differentiation capabilities. In addition, iMSCs have been shown to have better proliferation capabilities and advantages in tissue repair, immunoregulation and differentiation applications compared to tissue-derived MSCs. Nowadays, research on iPS cells is progressing rapidly, with at least 50 clinical trials underway in China, the United States, Japan and other countries. One of the most promising is the use of iPSC by China scientists to reverse type 1 diabetes, a world-first breakthrough that was just reported in Nature last month. In April this year, China scholars reported in Cell Discovery that iPSC technology was used to treat type 2 diabetes, and the patient had been cured. iPS cell-derived mesenchymal stem cells have strong immunoregulatory and regenerative properties. On October 15, 2024, German researchers published a research report entitled Unveiling the Immunomodulatory and regenerative potential of iPSC-derived mesenchymal stem cells and their extracellular vesicles in the industry journal Scientific Reports, revealing the immunoregulatory and regenerative potential of iPSC-derived mesenchymal stem cells and their extracellular vesicles.
Over the past two decades, multiple studies have shown that MSCs have strong clinical potential because of their powerful immunoregulatory effects through cell-to-cell interactions and the release of soluble factors and extracellular vesicles (EVs).
EVs are a class of small membrane-like nanoparticles that can carry a variety of biological molecules, including proteins, lipids, nucleic acids and small molecules. Interestingly, EVs play an important role in various physiological and pathological processes, such as cell-to-cell communication and immune regulation, and are thought to be involved in the progression of multiple diseases, including neurodegenerative diseases and cardiovascular diseases.
Recently, the use of MSC-derived EVs as therapeutic alternatives to MSCs has become increasingly popular because EV-based therapies can alleviate safety issues associated with the use of MSCs.
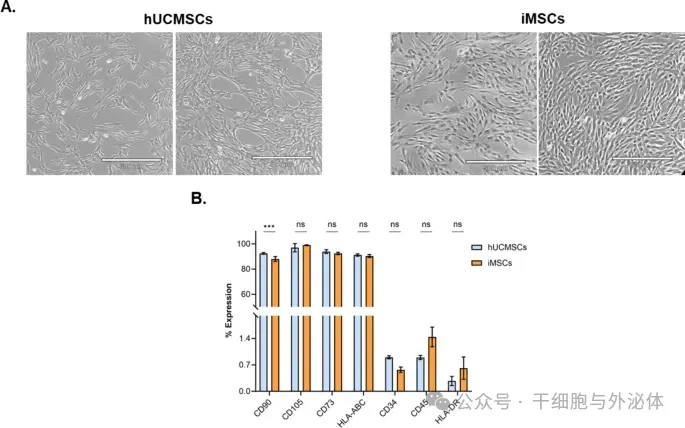
Figure: Characterization of primary hUCMSCs and iMSCs cells. (A) Representative forms of hUCMSCs (left panel) and iMSCs (right panel) in culture, with a scale of 500 μm. (B) Immunophenotypic characterization of hUCMSCs (blue bars) and iMSCs (orange bars), expressed as a percentage expression of a subset of classic cell surface markers, including CD90, CD105, CD73, HLA-ABC, CD34, CD45, and HLA-DR (n = 3). All data are expressed as mean ± SD. ns: not significant, ***: p < 0.001, by two-way analysis of variance and Sisak multiple comparison test.
iMSC therapy has more advantages than primary MSC therapy because iMSC is reported to be superior in producing EVs that regulate the microenvironment. The therapeutic effects of iMSC-derived EVs have been explored in many diseases, with the most thoroughly studied in wound healing, cardiovascular disease, and musculoskeletal pathology.
In order to comprehensively compare the therapeutic effects of iMSC and primary MSC, their functions and potential mechanisms must be clarified. To further explore this area and address existing gaps, German researchers have developed a comprehensive cell-based detection platform. The platform is designed to assess the immunomodulatory potential of iMSC and iMSC-EVs and includes several specific components to comprehensively assess their effects: T-cell proliferation and macrophage polarization assays, and the regenerative potential of iMSC-EVs in scratch assays.
The results demonstrate that the generation of induced pluripotent stem cell (iPSC)-derived MSCs (iMSC) provides an excellent opportunity to address most of the obstacles that limit the widespread use of MSCs for advanced cell therapies. In this study, the researchers compared the therapeutic effects of iMSC and its EVs with human primary umbilical cord derived MSC (hUCMSC) in vitro cellular assays. The results confirm that GMP-compliant iPSC can be used to generate iMSCs that meet primary MSC characterization criteria, and that these iMSCs release truly EVs.
Subsequently, the researchers found that iMSC effectively exerts its immunomodulatory potential, inhibiting T cell proliferation and inducing macrophage polarization towards an M2-like phenotype, just like hUCMSC. In addition, the study found that iMSC-EVs exhibited significant immunoregulatory properties similar to iMSCs cells, further demonstrating similar regenerative potential to hUCMSC-derived EVs. In addition, this study provides the first evidence of pro-inflammatory iMSC pretreatment, a feasible and compelling strategy to enhance the immunoregulatory effects of iMSC-EVs on human immune cells.