
In recent years, stem cells and regenerative medicine have become a new revolution in disease treatment, especially the use of stem cell drugs. Stem cell drugs refer to products based on living stem cells that are used as drugs for specific diseases.
Stem cells are believed to be the origin of human growth and development. The ability and relativity of stem cells to isolate, proliferate and transform have given rise to the field of use of stem cells for disease treatment.
Nowadays, stem cells have huge potential advantages in disease treatment (stem cell therapy). To date, more than 100 different diseases have been treated through stem cell transplants.
Hematopoietic stem cells (HSC) were first used in humans as early as the 1950s, indicating that stem cells can be used as drugs to treat diseases.
The clinical application of hematopoietic stem cells is rapidly increasing, and more and more diseases are being treated with hematopoietic stem cells. From the 1970s to the 21st century, all hematopoietic stem cell transplants (HSCTs) were for hematological malignancies. In recent years, hematopoietic stem cell transplantation has also been used for the treatment of solid tumors.
With the further identification of hematopoietic stem cells (including HLA typing), stem cells have been commercialized as transplant drugs around the world since the 2000s. However, due to the very low HLA matching rate in humans (about 1/100,000), the development of the hematopoietic stem cell drug business is slow.
Since 2012, stem cell drugs have developed into products based on mesenchymal stem cells (MSC). Similar to hematopoietic stem cells, in the first clinical application of MSC, MSC was administered via transplantation.
More and more studies have confirmed that MSCs have strong immunoregulatory capabilities and can regulate the host's immune system; moreover, they are less immunogenic than HSCs and other cells.
MSCs can be safely transplanted into patients without immunosuppression or HLA matching. Based on these results, MSC-based products are now being developed as drugs for patients with clear indications.
The first MSC-based stem cell drug was formally approved by the Food and Drug Administration of Canada in 2012. The drug is used to treat graft-versus-host disease (GVHD) associated with hematopoietic stem cell transplantation.
Stem cell drugs have now become an emerging and potentially important member of regenerative medicine. Cell drugs are a new member of stem cell therapy, personalized medicine and regenerative medicine. The development of stem cell drugs has had an impact and promotion on the stem cell industry and the pharmaceutical industry.
What are stem cell drugs?
A drug refers to any substance other than food that, when inhaled, injected, smoked, eaten, absorbed through a skin patch or dissolved under the tongue, causes physiological changes in the body.
In pharmacology, a drug (or pharmaceutical drug) is a substance used to treat, cure, prevent, or diagnose disease or promote health. According to this definition, a drug must meet certain criteria, such as having an indication for treating any disease and being an off-the-shelf product.
Thus, by definition, stem cell drugs are off-the-shelf products based on stem cells used to treat, cure, prevent or diagnose disease or promote health.
Stem cell drugs: from personalization to universal
Personalized medicine is a medical procedure that divides patients into different groups and tailors medical decisions, practices, interventions and/or products based on their expected response or risk of disease.
Stem cells provide a new approach to personalized medicine. In fact,"personalized medicine" using stem cells has become a popular term in the past 10 years.
So far, there are at least two ways to use stem cells in personalized medicine, as shown in the following figure.
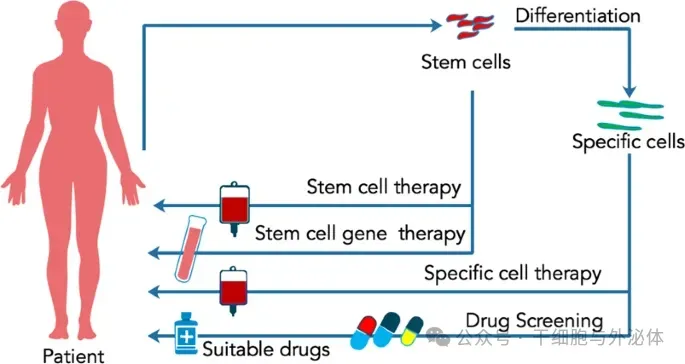
Picture: The application of stem cells in personalized medicine. Stem cells can be used in a variety of personalized medicine, including disease models for drug screening or evaluation, stem cell gene therapy for genetic correction, and stem cell therapeutic transplants.
In stem cell transplantation, the patient's autologous stem cells are used to treat his or her disease. However, the decline in the quality and number of stem cells due to aging is a major limitation of this approach.
However, after Nobu Yamanaka successfully induced the production of pluripotent stem cells (iPSC) from skin fibroblasts, scientists hope that personalized medicine can use autologous reprogrammed stem cells for wider clinical applications (picture below), such as diabetes, Parkinson's, stroke, tumors, etc.
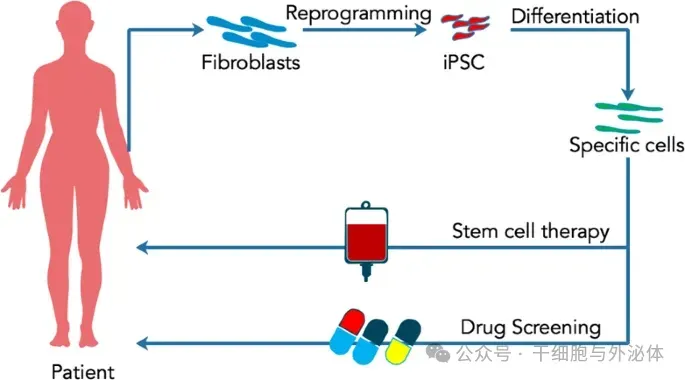
Picture: Induced pluripotent stem cells are used for personalized medicine. Fibroblasts originally isolated from the skin are then reprogrammed into induced pluripotent stem cells (iPSC). Before transplantation into patients, iPSC will further differentiate into specific cell types.
Stem cell drugs Inclusive Healthcare has become a new choice for stem cell therapy. Stem cell drugs can overcome all of the major limitations of autologous stem cells.
In particular, quality, quantity and price can be controlled. Based on the characteristics of ethical review and cell therapy, the need for personalized autologous cell medicine has become an important development trend and is a major form of medical treatment for regenerative medicine.
Medicines based on mesenchymal stem cells
Mesenchymal stem cells (MSC) are the most common stem cells in the human body. They are present in almost all tissues, but the most common sources are bone marrow, adipose tissue, cord tissue, cord blood and placenta.
Although MSCs from different organizations have common characteristics, they also exhibit different characteristics. Unlike other types of stem cells, MSCs have multiple functions; not only do they differentiate into multiple cell lineages, but they also produce a pool of cytokines and growth factors to perform immune regulation and promote injury healing and tissue regeneration.
MSCs are suitable for clinical applications in stem cell therapy due to their multi-lineage differentiation potential. Stem cells can differentiate into functional cells in vitro or in vivo to replace aging or damaged cells.
In most cases, MSCs are used as a source of stem cell drugs. The two main therapeutic mechanisms mediated by stem cell drugs are immune regulation and paracrine/endocrine effects. Immunomodulation is the most common mechanism in today's commercial stem cell drugs; approximately 80% of stem cell drug products act through immunomodulation.
This means that the host immune system can be regulated through indirect or direct interactions between stem cells and host immune cells.
The second major mechanism of action of MSCs is related to growth factors produced by stem cells. MSC can produce a range of growth factors and cytokines that have biological effects on other cells.
MSC can produce factors that stimulate host stem cells, inhibit apoptosis, and stimulate angiogenesis. Some cytokines play a paracrine role, while others may have an endocrine role.
Drugs based on induced pluripotent stem cells
The field of stem cell biology is developing rapidly due to the astonishing development of reprogramming technology. The ability to restore somatic cell pluripotency through ectopic co-expression of reprogramming factors creates powerful new opportunities to simulate human disease and holds promise for personalized regenerative cell therapy.
After decades of constraints, cell biology has become a booming research field as it has achieved a long-cherished wish to successfully isolate pluripotent stem cells from patient cells.
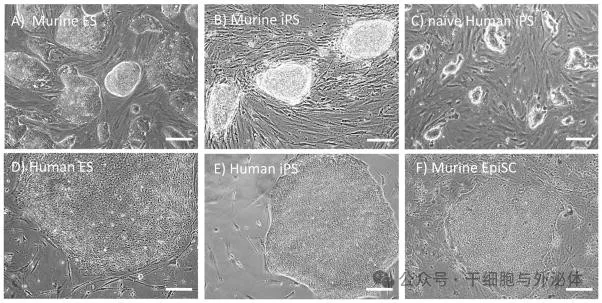
Figure: Mouse embryonic stem cells ES ( a ) and induced pluripotent stem cells iPSC ( b ) cells form dome-shaped, refracting colonies.
Induced pluripotent stem cells (iPSC) are derived from skin or blood cells that have been reprogrammed back to an embryo-like pluripotent state, allowing the development of an unlimited source of any type of human cells needed for treatment.
iPSC has broad application prospects in cell therapy, disease modeling, drug discovery and toxicological screening.
In recent years, regenerative medicine using iPS cell technology has made significant progress. iPS cell-based therapies or therapies that are about to be applied clinically, including therapies for ophthalmology, neurology, cardiology, hematology, cartilage and metabolic diseases.
For example, induced pluripotent stem cells can be induced to become beta islet cells to treat diabetes, blood cells can create new blood for leukemia patients that does not contain cancer cells, and neurons can treat neurological diseases.
In the next few years, regenerative medicine based on iPS cell technology will be further popularized.
The future of stem cell drugs
In recent years, stem cell therapy research has evolved from using entire stem cells to using components derived from stem cells. These ingredients include stem cell extracts, microvesicles and exosomes, all of which exhibit various biological activities.
For example, exosomes from MSCs have similar functions to entire MSCs, including repairing tissue damage, suppressing inflammatory responses, and regulating the immune system.
Research has shown that exosomes from human stem cells can accelerate skin wound healing by optimizing the properties of fibroblasts. Similar to MSCs, extracellular vesicles (EVs) from MSCs can regulate the immune system.
Exosomes from stem cells can also affect other systems and organs, such as the cardiovascular system, kidneys, liver, nervous system and musculoskeletal system. In the cardiovascular system, exosomes are considered cardioprotective agents; they have been shown to have pro-angiogenic effects and are involved in reducing apoptosis and cardiac fibrosis.
Clinical studies have demonstrated that a single intravenous injection of exosomes can effectively enhance cardiac function and geometry after myocardial infarction due to bioenergetic reconstitution, reduction of oxidative stress, and activation of pro-survival signals (enhanced PI3K/Akt signals).
In terms of kidneys, studies have shown that MSC-based exosomes can effectively treat acute kidney injury. In addition, MSC-derived exosomes can also be used to treat fibrotic liver disease.
In certain musculoskeletal system diseases, exosomes can trigger the differentiation of MSCs into osteoblasts and stimulate skeletal muscle regeneration by enhancing muscle production and angiogenesis.
In the nervous system, MSC-derived exosomes have been evaluated in the treatment of nervous system and neurodegenerative diseases; they have been shown to enhance angiogenesis and neurogenesis, reduce inflammation, and improve spatial learning and sensorimotor functions.
To sum up, the above findings herald the arrival of a new era of stem cell therapy (i.e., stem cell drugs). Here, the stem cell components (mRNA, proteins and peptides) in the microvesicles or exosomes can be more effective than the entire stem cells.
Of course, the advantages of stem cell drugs include reduced immunogenicity and ease of processing, storage and delivery. Drugs that do not contain stem cells may play a potentially important and emerging role in regenerative medicine.
Stem cell drugs are a new member of medicine produced using stem cells. Countries have approved more than ten stem cell drugs for clinical use. These products may contain living hematopoietic stem cells or mesenchymal stem cells.
Stem cell drugs have become a promising new platform in the global field of stem cell therapy due to their advantages of reduced immunogenicity and ease of processing.
As a new pharmaceutical product, stem cell drugs are expected to make huge contributions to the pharmaceutical and medical industries in the near future. In terms of clinical applications, in addition to stem cell drugs containing living and whole stem cells, new stem cell drugs containing stem cell components (such as extracts, exosomes and vesicles) are under development and are expected to be available soon.