
Large-scale production of induced pluripotent stem cells (iPSC) is critical to treating multiple clinical indications. However, growing enough iPSC for clinical use poses significant challenges, including their sensitive pluripotent state and reliance on supporting substrates. Developing stem cell bioprocess strategies that can be scaled up and meet clinical needs requires incorporating methods that can monitor intrinsic markers of cell differentiation status, growth status, and activity in real time. In addition, appropriate cell culture models to support high-quality suspended stem cell growth in suspension are key to industrial scale. In this article, we will provide an overview of cell culture media, suspension models, and monitoring technologies that can maintain the quality and pluripotency of iPSC during induction, expansion, and production.
Recent developments and challenges in stem cell production
Cell therapies involving stem cells, such as tissue transplants or drug discovery, are used to treat a variety of clinical indications, mainly in the fields of oncology, heart disease, immune and neurological diseases. Therefore, a major focus in the field of stem cell research is to advance cell growth strategies while maintaining control of cell differentiation. Traditionally, embryonic stem cells (ESC) have been an ideal cell type for cell therapy because of their inherent pluripotency, the ability of cells to differentiate into any specialized cell type. The discovery of these cells provides huge opportunities for regenerative medicine and the treatment of various pathological diseases. Regardless of the stem cell origin, ESCs must undergo self-renewal during proliferation to maintain pluripotency, during which differentiation into specific cell types is inhibited.
In 2007, Yamanaka and colleagues led a major technological breakthrough in the field of stem cells, successfully transforming human somatic cells into stem cells with similar gene expression profiles and pluripotency to human ESC (hESC). These cells are called human iPSC (hiPSC). By avoiding the use of embryos to extract stem cells to address ethical issues, iPSC is a more favorable research and clinical application platform. Given its inherent self-renewal capabilities, pluripotency and relatively low immunogenicity, iPSC represents a very promising, unlimited patient-derived cell for human genetic disease modeling and toxicology research, which reduces the overall costs and associated risks of drug development and clinical trials. Due to its multifaceted capabilities, iPSC technology remains a promising scientific tool for personalized cell therapy and regenerative medicine.
Current clinical cell therapy and human tissue regeneration require 10^8-10^10 clinical grade stem cells and are cultured in accordance with current Good Manufacturing Practice (cGMP) processes. However, scaling up cell culture to the necessary level remains a major challenge due to its reliance on supporting substrates and the pluripotent state to which pluripotent stem cells are sensitive. The final quality of iPSC during harvest depends on the metabolic state of the cells; more specifically, maintaining pluripotent state and self-renewal is critical to the production of clinical grade iPSC. Therefore, methods of monitoring intrinsic markers of cell differentiation status, growth status and activity are most useful for large-scale production. In addition, existing platforms are being optimized to meet cGMP standards to effectively scale up bioprocesses into clinical production environments. In this article, we will highlight the latest advances in iPSC cell culture methods, including media, suspension models and monitoring technologies, which have maintained the quality and pluripotency of iPSC to a certain extent to meet the needs of clinical production. In addition, we will discuss future technologies that have the potential to improve iPSC bioprocess efficiency and yield.
Isolation of iPSC from a heterogeneous cell population
Many signals can activate stem cell differentiation, so subtle changes in cell culture conditions or stress on cells can lead to heterogeneous differentiation of cell populations. This is a serious safety issue because differentiated cell contamination can cause potential tumor or teratoma formation in cell transplant recipients. However, spontaneous stem cell differentiation can occur during cell culture, as observed when mesenchymal stem cells (MSC) are cultured for extended periods in extracellular matrix. To regulate this type of reaction and preserve PSC for future applications, cell sorting methods to separate pluripotent and differentiated cells have been used. Fluorescence activated cell sorting (FACS) and microwell adhesion are popular high-throughput methods for separating cells based on defined pluripotency characteristics, and the selectivity of pluripotency labeled positive stem cells is enhanced by cell culture conditions. For iPSC, when a small molecule mixture of four inhibitors (SMC4 medium) was added, a 55-fold increase in SSEA4/tra181-positive iPSC clones was observed after sorting compared to cell-derived clones sorted in traditional reprogramming medium. Although FACS is highly automated and standardized, sorting individual cells to produce stable pluripotent cell clones is still a labor-intensive and time-consuming task. Therefore, methods capable of producing pluripotent cell populations as pool culture are preferred because pools can maintain long-term stable expression of pluripotent markers. To this end, a magnetically activated cell sorting (MACS) method using cell-surface labeled antibodies has been used to generate iPSCs pools. In this case, after a round of heterogeneous cell pool sorting with TRA-1-60 and SSEA4 antibodies, the number of TRA1 -60 and SSEA4 positive cells increased by 28% and 11%, respectively. Additional rounds of MACS further enriched the cell population expressing pluripotency markers. In general, MACS is a better method than FACS because it can be performed easily and quickly on multiple samples simultaneously while applying only a small shear stress to the cells.
Although cell sorting methods are effective in characterizing stem cell populations based on morphology and surface markers, the current widespread use of cell sorting methods to isolate animal or clinical research-grade pluripotent stem cells remains hampered. This is mainly due to the high cost of GMP-grade antibodies and the limited availability of clinic-grade FACS instruments and expertise. Therefore, in future clinical trials of cell therapy, a scalable platform is needed to generate reliable, uniform and safe clinical grade pluripotent stem cell populations.
Development of the best iPSC medium and medium
A key step in controlling the quality of iPSC during the expansion process is the use of well-characterized materials in biological processes, where cell culture media and substrates play a key role. Cell culture media is critical to maintaining healthy, proliferating cells by providing a good balance of nutrients, minerals and pH. Cell culture substrates are used as scaffolds for cell adhesion and proliferation, and the substrates are usually wrapped with feeder cells or growth support factors to further enhance cell adhesion and growth. The use of fully characterized cell culture systems is critical for well-controlled bioprocesses, especially for the production of clinical grade biological samples. Over the past decade, iPSC media formulations and substrates have been significantly developed by considering signaling pathways that help maintain the pluripotency of iPSC cell lines and overall process amplificability.
When it comes to designing media for iPSC, one strategic approach is to identify the intrinsic growth factors involved in pluripotency dependent signal transduction pathways. There are many ways to regulate the level of stem cell pluripotency genes, such as the activating cascade of transforming growth factor (TGF)-ß superfamily, the receptor tyrosine kinase signaling pathway [downstream of basic fibroblast growth factor (bFGF)], pathways involving insulin-like growth factor (IGF), etc. Interestingly, the proteins and growth factors in mESCs sufficient to maintain pluripotency (i.e., bone morphogenetic protein (BMP) and leukemia inhibitory factor (LIF) ) are different from hESCs. Pluripotent stem cells may also require different growth factors to maintain pluripotency and self-renewal. For example, bFGF has been identified as a key additive in maintaining hESC self-renewal in vitro, with concentrations ranging from 40 ng/ml to 100 ng/ml in feeder-free cultures. Typical Wnt/b-catenin signaling is also associated with hPSC self-renewal, although addition of Wnt3a alone is not sufficient to maintain undifferentiated hESCs in the absence of feeder cells. Given that these metabolic studies were conducted on hESCs rather than iPSCs, more in-depth characterization of iPSCs is needed to design media and consider the unique requirements of each new cell line developed for clinical applications.
By providing specific stem-supporting factors and creating an extracellular matrix (ECM)-rich environment, feeder cell-based substrates prevent spontaneous differentiation of stem cells and improve attachment of ESC and/or iPSC. The most commonly used feeder cells to support PSC are proliferation-inactive mouse embryonic fibroblasts (MEFs) because they produce a variety of proteins critical to the maintenance of pluripotency, such as TGF-beta1, activin A, BMP-4, polytrophic factors (heparin-binding growth factors), etc. However, due to the limited proliferation ability of feeder cells, the efficiency of supporting iPSC pluripotency after repeated passage is reduced, and a high risk of contamination during the iPSC isolation process can lead to related technical challenges arise when large-scale production of iPSC under feeding conditions. In addition, animal-derived feeding substrates may increase the risk of zoonotic pathogens and unknown viruses migrating to host cells, leading to immune system rejection. Therefore, the iPSC culture method focuses mainly on transitioning to an animal component-free and cell-free (known as "feed-free") culture system through the use of ECM proteins, conditioned media, or synthetic biomaterials.
With the development of media, iPSC cell culture substrates have gradually reached cGMP standards in the past ten years. A recent iPSC study showed that long-term use of animal-derived sera and xeno-containing molecules affects cell morphology, amplification potential, gene expression, and cytokine profiles. This led to the development of xeno-free medium (XFM) formulations, followed by animal-derived component-free (ACF) media to support iPSC expansion. Among the developed ACF medium types, Essential 8TM (Thermo Fisher Scientific) medium is the most used basic medium in iPSC culture because it contains the eight elements most necessary for stem cell proliferation: DMEM/F12, L-ascorbic acid, magnesium phosphate, sodium selenide, FGF-2, insulin, NaHCO3, and transferrin, TGF-β1 or Nodal.
The development of feeder iPSC amplification methods promotes the possibility of automated production in the future. In fact, using the CompacT SelecTTM cell culture system, large-scale automated production of undifferentiated iPSC under feed-free conditions is feasible. In this case, polymer hiPSC automatically passaged using chemically defined medium (CDM) containing Activin A and FGF-2 retained its characteristic morphology and expression of pluripotency markers. Biomaterials are also being explored as enhanced substrates for feeder iPSC culture systems. For example, one study found that hydrogel-based substrates have optimal elasticity (25 kPa), allowing cells to remain pluripotent. Further studies showed that the double-stranded in vitro connectin-derived oligopeptides grafted onto a hydrogel (storage modulus of 25 kPa) support long-term growth of hESCs and iPSC for more than 10 generations. ECM, such as fibronectin, laminin, and vitronectin, or oligopeptides from ECM, have specific cell-binding domains, making them an important component in supporting the growth of iPSC in feeder matrix-free systems. 3D bioprinting and cell/tissue printing technologies also unlock possibilities for future process scale-up and automation. Recently, the effectiveness of cell printing technology was demonstrated when iPSCs were acclimated and expanded on feeding-free chitosan or polyurethane membranes coated with fibronectin. In this study, iPSC embedded in a heat-sensitive polyurethane (PU) hydrogel matrix showed higher activity. However, further optimization of polymer-based feedless substrates is necessary because the pluripotency markers (i.e. OCT4 and NANOG) of PU hydrogel cultures are different from control MEF feeder layer cultures.
Cell culture dynamics determine not only the ultimate behavior of iPSC, but also the cost of the entire biological process. Although the feasibility of large-scale growth of hPSC in 2D static cultures has been demonstrated in disposable multilayer culture vessels with the ability to monitor and feedback control pH and dissolved oxygen (DO), large-scale production of hPSC on 2D or 3D static substrates remains a cost, labor and space-intensive method. Static culture conditions are also known to cause unfavorable gradients of medium composition, metabolic wastes, paracrine factors and gases. The main consensus is that dynamic suspension culture is the best method to achieve the density of pluripotent stem cells required for clinical applications, and new strategies for expanding suspended pluripotent stem cells that are efficient and scalable are currently being actively developed. For example, successful substrate-based research can be used to design the optimal suspension culture model.
Key considerations for iPSC suspension methods (aggregates, microcarriers, and microcapsules)
The substrate-less iPSC suspension culture system overcomes the challenge of limited scalability of static substrates while supporting iPSC growth and pluripotent states. Due to the adherent nature of iPSCs, 3D aggregates (or spheres) form spontaneously when they are seeded and expanded in suspension systems. iPSC aggregates are very similar to embryoid bodies (EBs) and can therefore serve as a useful means of direct lineages differentiation after amplification. The growth and pluripotency of iPSC aggregates mainly depend on the microenvironment, aggregate size distribution, and cell culture tank size. Some studies have optimized the culture conditions so that hiPSCs can be expanded as undifferentiated suspension cell aggregates for more than 10 generations under E8TM feeding conditions in spinner bottles. Since then, stirred suspension cultures have been expanded to a 3000-ml disposable bioreactor (1000-ml working volume) to produce large numbers of hiPSC aggregates (up to 2x10^9 cells) while retaining expression of pluripotent state markers, including TRA-1-81, SSEA-4, OCT4 and SOX2.
However, there are limitations in expanding iPsc aggregates in dynamic suspension culture. Compared with static suspension culture, the amplification rate was reduced and the aggregate size was formed unevenly. If not controlled, the formation of large clumps of cells (>800 um) can lead to a slowdown in cell activity, spontaneous differentiation, nutrient and oxygen diffusion gradients, and the overall expansion process. By optimizing the paddle type and stirring speed, the aggregate size in iPSC culture can be controlled. An example of a new bioreactor system is the Vertical Wheel Bioreactor (VWBR), which expands iPSC while reducing the size of aggregates, where agitation is provided by a vertical impeller, allowing efficient homogenization of the vessel. Using this system, aggregates with an average diameter of 350 um were produced (maximum density of 2.3x10^6 cells/ml) while maintaining pluripotency.
Media additives can also affect the formation of aggregates. For example, short-term treatment with retinoic acid (RA) can further maintain pluripotency during hiPSC aggregate expansion. Retinoids support iPSC self-renewal by increasing the expression of pluripotent dependent transcription factors (Nanog and Oct4) and activating the phosphatidylinositol-3 kinase (PI3K) signaling pathway. In addition, the addition of Rho-related coiled coil kinase (ROCK) inhibitor Y27632 to chemically defined media can promote the formation of iPSC aggregates starting with single cell seeding in multiple suspension tank types. Coiled coil kinase inhibitors support the survival of stem cell aggregates by reducing dissociation-induced apoptosis and improving cloning efficiency. However, recent studies have shown that long-term exposure to ROCK inhibitors alters the metabolism of iPSC. Therefore, medium addition, inoculation strategy and bioreactor settings are key parameters for optimizing bioprocess yields. Through further optimization, large-scale production of iPSC aggregates for sufficient clinical applications can be achieved. Under this model, both the nutrient transport mechanism and the size distribution of aggregates need to be improved to maintain the pluripotency and vitality of cell aggregates at high densities.
microcarrier
Efforts to strengthen stem cell culture and expansion in bioreactor systems have made progress in the implementation of microcarriers. The advantage of microcarriers is that they provide a surface area to support the growth and attachment of PSC while maintaining the advantages of a dynamic suspension culture system. Microcarriers can be used for cell seed preparation or, if biodegradable, ultimately used to transport cells to the desired damaged tissue during treatment. Various biodegradable materials are commonly used to produce microcarriers for cell line expansion, including dextran, collagen, gelatin, polylactic-glycolic acid (PLGA), polyl-lactic acid (PLLA), polystyrene (PS) and hydroxyapatite (HA). Similar to larger static substrates, microcarriers provide further enhanced support for the growth of PSC through appropriate linkage of their surface charges and when encapsulated by ECM proteins such as vitronectin, fibronectin, and laminin. Stirred microcarrier culture systems have been shown to promote the expansion and scale-up of adhesion-dependent stem cells, while providing the necessary tools to monitor and control stem cell health and differentiation. Increase PSC production by enhancing oxygen supply and mass transport of metabolites and reducing microenvironmental toxicity.
However, when using microcarriers to amplify hiPSC, there are some issues to consider. Limitations of microcarriers depend on their diameter (100- 400um), density (usually-1 g/ml) and chemical composition, and they can affect cell attachment and thus expansion capabilities. Due to the limited surface area of the microspheres, the peak density of hiPSC appears to be limited by the ratio of microspheres to cells. Cells adhered to the microcarrier microspheres also require enzymatic hydrolysis and filtration, which may sacrifice the vitality and pluripotency of the cells, depending on the method used. To alleviate this problem, biodegradable microcarriers are under development. Compared with traditional PS microcarriers, the development of non-native soluble microcarriers has recently made great progress, greatly improving the recovery rate of cells in spinner flasks.
Stirred bioreactors also exert hydrodynamic shear stress on the microcarrier microspheres, which affects the overall health and pluripotency of hiPSC. This can be overcome by optimizing bioreactor process parameters (i.e. agitation rate) to minimize the impact of shear stress on iPSC. For example, Gupta and colleagues demonstrated that long-term attachment, pluripotency, and expansion of iPSC on microcarriers in spinner flasks rely on maintaining an optimal agitation speed of 25 RPM. This parameter optimization method needs to be applied to larger-scale iPSC expansions (i.e. bioreactors).
The cost of manufacturing microcarrier microspheres is another consideration, as the process can become extremely expensive depending on the materials and additives used. Therefore, it is important that the microcarrier material be cost-effective, if possible-recyclable, and sterilizable after each production run. Using well-controlled bioprocess systems, microcarrier-based iPSC culture methods are being developed for the continuous expansion and recovery of human iPSC in cell therapy and tissue engineering.
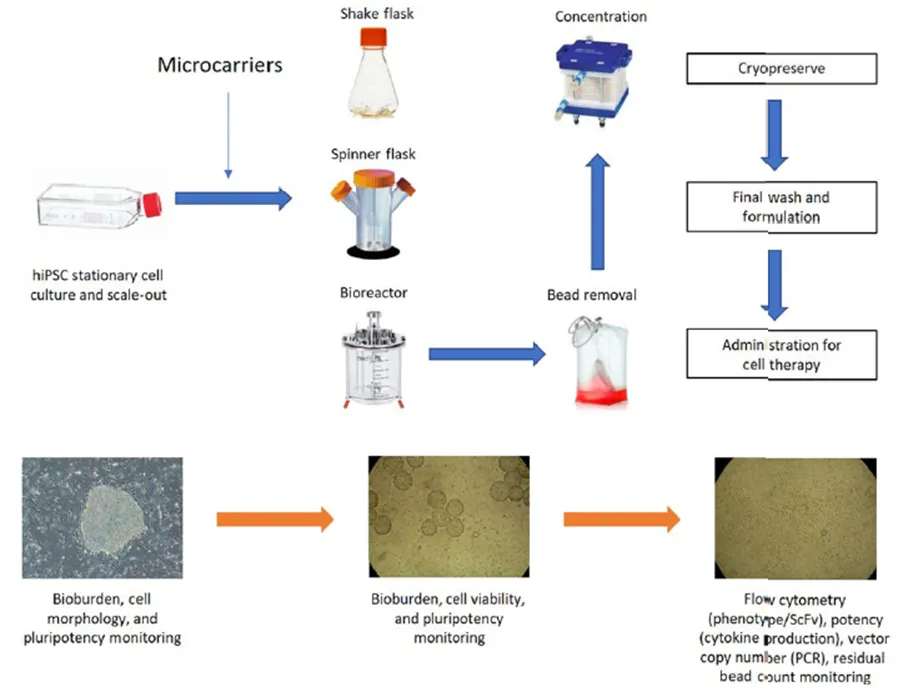
Research progress on biological processes for preparing human induced pluripotent stem cells (hiPSC) using microcarriers. Typical processes include static culture, scale-up process, downstream process and preparation. Microcarriers are usually introduced during the scale-up process and removed during the downstream process.
microencapsulation of
Microencapsulation is different from microcarriers that attach cells to the surface of microspheres. Microencapsulation involves trapping cells in spherical capsules through which nutrients, oxygen and other growth factors needed for cell growth can be diffused. Spherical capsules are composed of semi-permeable materials or membranes that protect cells from agglomeration and shear forces in suspension culture systems. Biomaterials used to create microcapsules include alginate, agarose, nylon, colloids, polystyrene, acrylates, polylysine-alginate hydrogels, cellulose acetate-ethyl cellulose, and polyester films. Cells are usually captured by emulsification or extrusion to form protective tubes that allow cells to proliferate. In general, polymer microcapsules have certain advantages over gel microcapsules, including biocompatibility and GMP compatibility. The capsule material can accommodate more cells per unit volume, and due to the presence of liquid cell suspension in the space within the capsule, inter-particle diffusion restrictions in the polymer capsule are less severe.
Stem cells cultured in the microcapsule system can both maintain pluripotency and be induced to differentiate, depending on the composition of the capsule and the growth factors present in the microenvironment. Microencapsulation technology faces similar shortcomings to microcarriers in large-scale production, namely manufacturing costs and limited surface area. In addition, some stem cell types, such as hMSC, have difficulty proliferating when encapsulated (e.g., in alginate) without added peptides or proteins, such as fibronectin, which improves cell attachment. In contrast, if cell attachment is enhanced, recovering wrapped cells during harvest may pose a more difficult challenge.
Original text:A.Polanco, B.Kuang, S.Yoon, Bioprocess Technologies that Preserve the Quality of iPSCs. Trends in Biotechnology, 2020.