
In the biomedicine industry, cell therapy is ushering in rapid development momentum, among which universal cell therapy has become a key development direction due to its excellent accessibility and economic benefits. With its unique advantages, such as multi-directional differentiation potential, infinite amplification, and high gene modification efficiency, iPSC technology provides an ideal technology platform for this therapy. In 2024, nearly 35 domestic iPSC companies entered the market, promoting the rapid progress of universal cell therapies. With the help of the capital market, many companies completed a new round of financing in 2024, providing research and development of iPSC therapies. Strong financial support has been injected into the commercialization.
It is estimated that the global iPSC market will be approximately US$1.71 billion in 2023, and the compound annual growth rate is expected to be 10.21% from 2024 to 2030. With the continuous maturity of technology and the optimization of the policy environment, the industrialization process of iPSC technology has accelerated significantly. Many domestic and foreign companies have successfully pushed their products to the clinical stage, and there is little gap in progress at home and abroad. This article aims to provide a summary and analysis of the world's leading iPSC companies.
1 A quick overview of the global clinical iPSC product pipeline
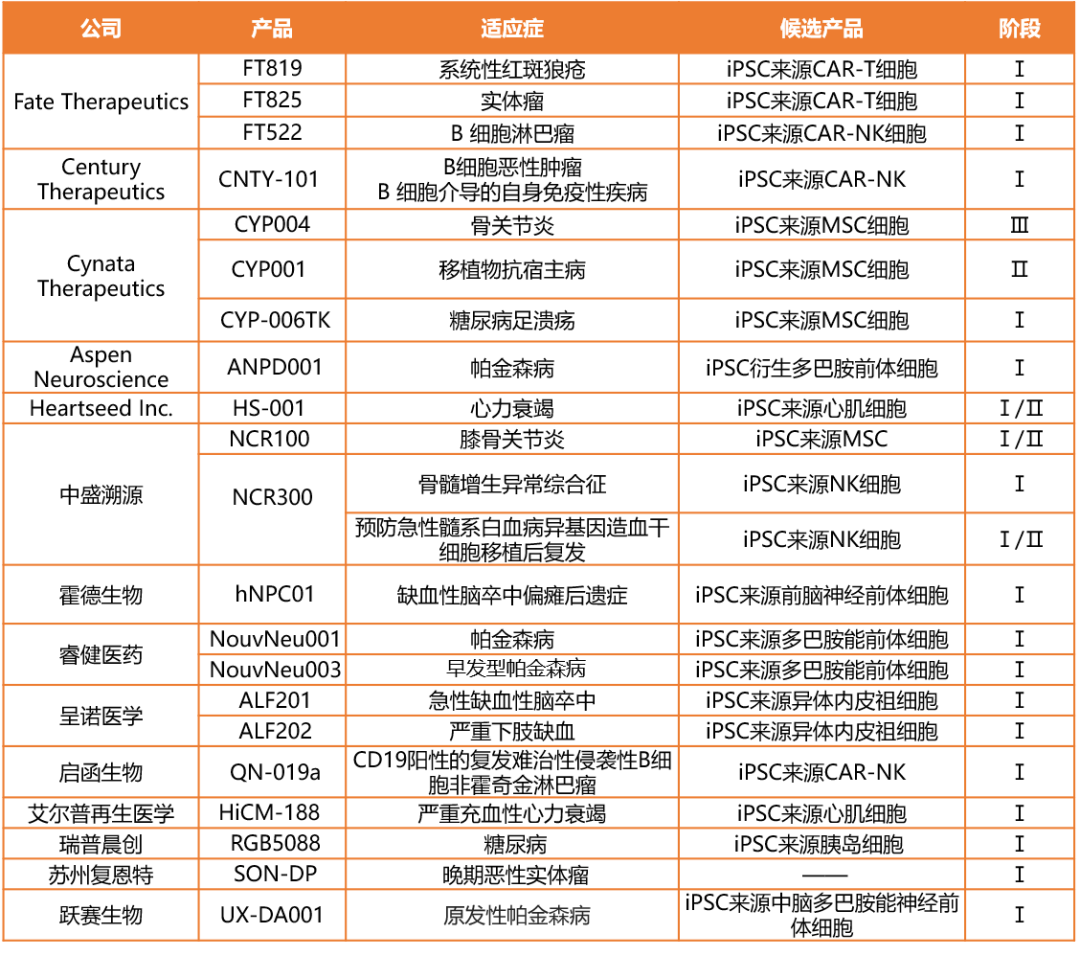
2 Company inventory
Fate Therapeutics
Fate is a U.S. clinical biopharmaceutical company dedicated to developing multiple engineered iPSC-derived immune cell therapies for cancer and autoimmune diseases. The product line includes iPSC-derived natural killer (NK) cells and T cells. Currently, there are three products: FT819 (iPSC-derived CAR-T) for the treatment of systemic lupus erythematosus (SLE), FT825 (iPSC-derived CAR-T) for the treatment of solid tumors, and FT522 (iPSC-derived CAR-NK) for the treatment of B-cell lymphoma.
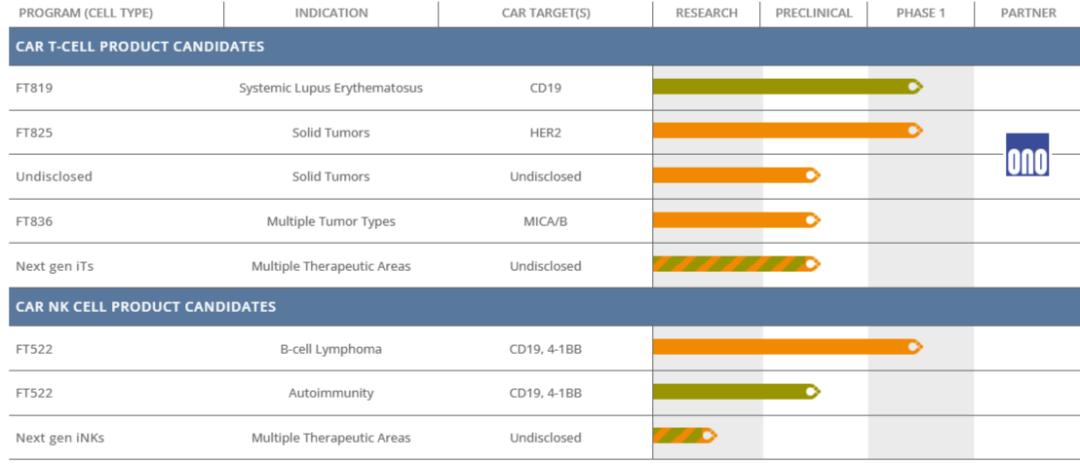
The latest FT819 clinical phase 1 data released in September this year showed that three patients treated in the first dose group showed rapid and persistent B cell depletion and had good safety profile. The first subject met the systemic lupus erythematosus definition of response (DORIS) response criteria after a single infusion and maintained low disease activity status (LLDAS) during the six-month follow-up period. As of the six-month follow-up, the patient remained in clinical remission and did not require any immunosuppressive treatment.
FT522 (iPSC-derived CAR-NK) is currently conducting a multi-center, Phase 1 study on B-cell lymphoma, and plans to further expand clinical research to autoimmune diseases.
Century Therapeutics
Century is an innovative American biotechnology company dedicated to developing iPSC-derived cell therapies in the fields of oncology, autoimmune diseases, and inflammatory diseases. Currently, its CNTY-101 (iPSC-derived CAR-NK) product is in clinical phase 1 and is targeted for two indications: autoimmune diseases and tumors.
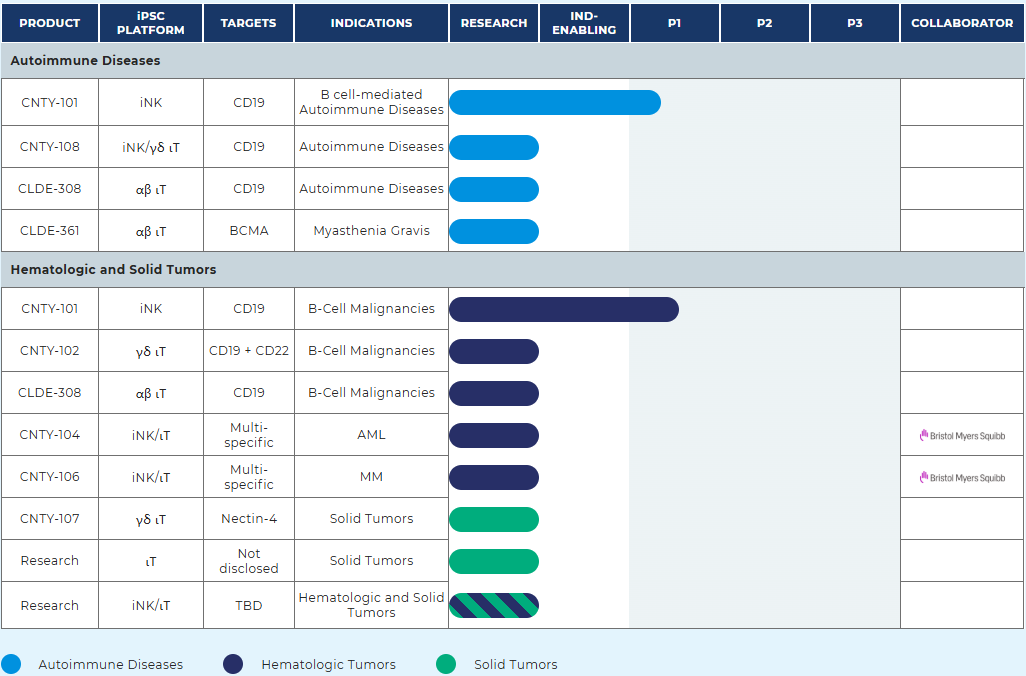
In May this year, the company released two preclinical data. In vitro data showed that its main candidate drug CNTY-101 can induce CD19-specific B cell lysis, demonstrating the potential for treating B cell-mediated autoimmune diseases, including systemic lupus erythematosus (SLE). In addition, preclinical data further confirms that the use of synthetic ligand CD300a agonists is expected to protect allogeneic cell therapies from killing by NK cells.
Recently, the company announced the termination of a major cooperation agreement with pharmaceutical giant Bristol-Myers Squibb (BMS). This collaboration, which focuses on developing new therapies for hematological malignancies, will officially end on March 12, 2025.
Cynata Therapeutics
Cynata is an Australia stem cell and regenerative medicine company that uses CymerusTM technology to produce iPSC-derived mesenchymal cell (MSC) products. Currently, the company has three iPSC-derived MSC cell (iMSC) products in clinical practice. Among them, CYP-004 (iMSC) is the world's first iPSC-derived cell therapy product to enter clinical phase 3 for the treatment of osteoarthritis. Two other iMSC products have entered the clinical phase 2, namely CYP001 for the treatment of graft-versus-host disease (GvHD) and CYP006TK for the treatment of diabetic foot ulcers (DFU).
In February this year, the company announced the preliminary analysis results of the wound surface area of the first batch of 16 patients in the Phase 1 clinical trial of CYP-006TK for the treatment of diabetic foot ulcers (DFU). The results showed that after 10 weeks of treatment, the median percentage of wound surface area reduction in patients in the CYP-006TK group (n=8) was 87.6%, compared with 51.1% in the control group (n=8).
In May this year, the company released two-year follow-up data of CYP-001 in SR-aGvHD patients. The results showed that the OS of CYP-001 reached 60% in two years, and it had good safety and tolerability.
Heartseed Inc.
Headquartered in Tokyo, Japan, Heartseed is committed to developing and commercializing cardiac muscle reconstruction therapies developed by Keiichi Fukuda and his team at the Department of Cardiology at Keio University in Tokyo, Japan. At present, its product HS-001 (iPSC-derived cardiomyocytes) has advanced to Phase 1/2 clinical treatment for heart failure.
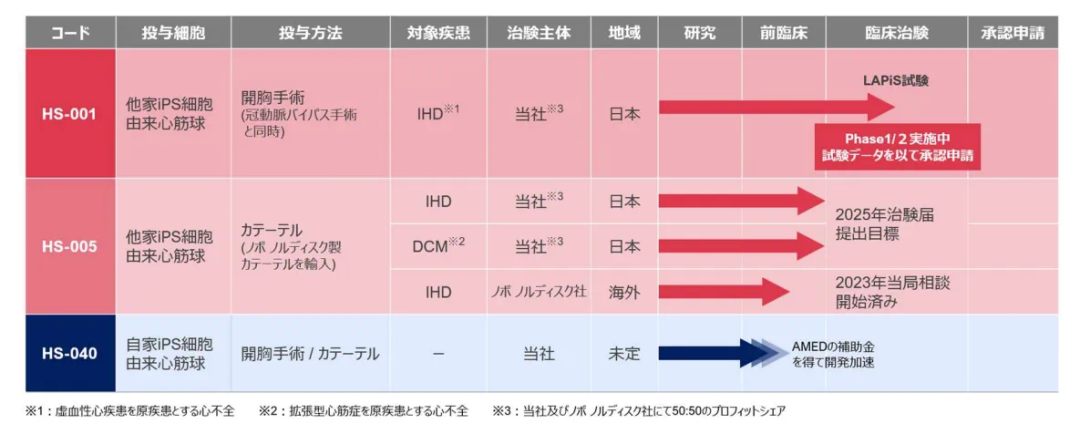
Clinical data released by the company in May this year showed that administration to five patients with advanced heart failure caused by ischemic heart disease in the low-dose group (50 million cardiomyocytes per patient) had been completed, and no dose-limiting toxicity or safety issues affecting the continuation of the trial were observed. The Safety Monitoring Committee (SMC) has recommended entering the high-dose group (150 million cardiomyocytes) to further evaluate the effectiveness and safety of HS-001.
In July this year, the company announced its listing on the Tokyo Stock Exchange (TSE), with a market value of 34.755 billion yen (approximately 1.646 billion yuan) on the day of listing.
Aspen Neuroscience
Aspen Neuroscience is headquartered in San Diego, California and co-founded by Dr. Jeanne Loring, a stem cell biologist at the Scripps Research Institute, and aims to develop autologous induced pluripotent stem cell (iPSC)-derived cell therapies to address highly unmet medical needs. Diseases. Currently, ANPD001 (iPSC-derived dopaminergic neurons) is in clinical stage 1/2a for the treatment of sporadic Parkinson's disease.
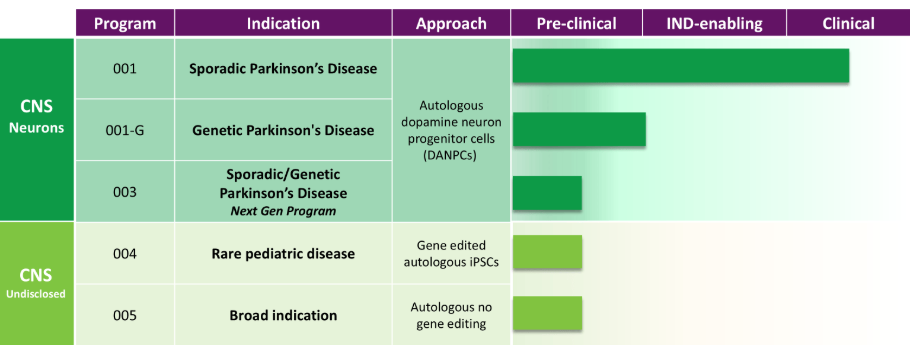
In September this year, the company announced that ANPD001 had completed the first batch of patients and demonstrated reasonable safety.
In October this year, the company announced a partnership with Cell X Technologies to increase the scale of its own iPSC production.
Anhui Zhongsheng Traceability Biotechnology Co., Ltd.
Zhongsheng Traceability is the earliest company in China engaged in the development of iPSC-derived cell therapy products. It was founded by Dr. Yu Junying, one of the international pioneers of iPSC technology, and is committed to developing widely available cell therapy products. The company focuses on key medical fields such as anti-inflammatory repair, tumor immunity and regenerative medicine. Currently, NCR100 (iPSC-derived MSC) has advanced to clinical stage 1/2 (the first and only approved clinical iMSC in China), NCR300 is in clinical stage 1 for the treatment of myelodysplastic syndrome (the first approved clinical iNK product in China), and NCR300 is in clinical stage 1/2 for preventing recurrence of AML after allo-HSCT.
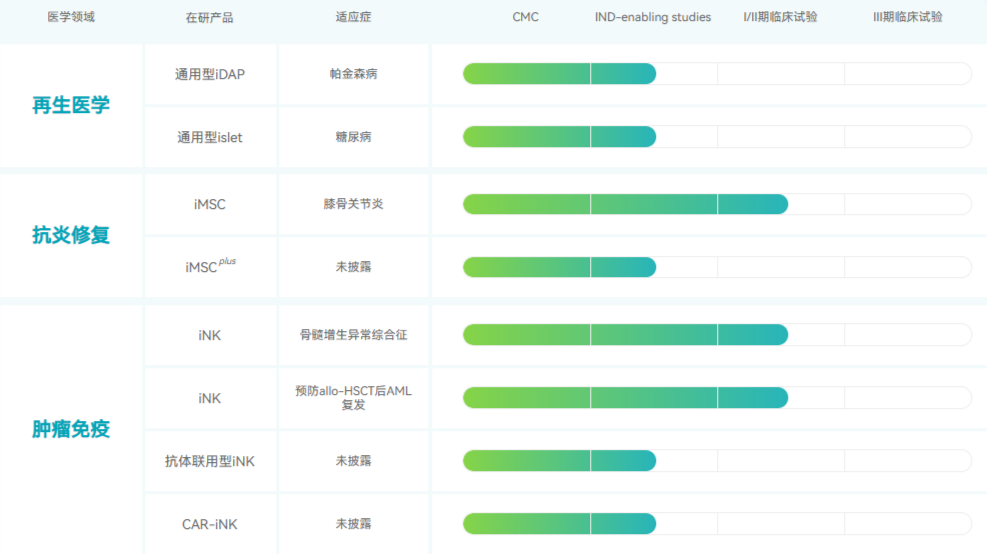
In November this year, the company announced that it had completed the first round of Series B financing of 150 million yuan. The funds will be used to accelerate the clinical development of its various clinical pipelines in the field of iPSC cell therapy and the commercialization of subsequent products, and promote the development of the field of cell therapy.
Zhejiang Huode Bioengineering Company Limited
Huode Bio was co-founded by neurology and stem cell scientists at Johns Hopkins University in the United States. It focuses on the development of universal cell therapy products derived from iPSC. It has established a CMC platform for iPSC cell products, a full-suspension automatic production process and a variety of innovative analytical methods. Currently, hNPC01 (iPSC-derived pre-cranial nerve precursor cells) is in clinical stage 1 for the treatment of hemiplegia sequelae due to ischemic stroke.
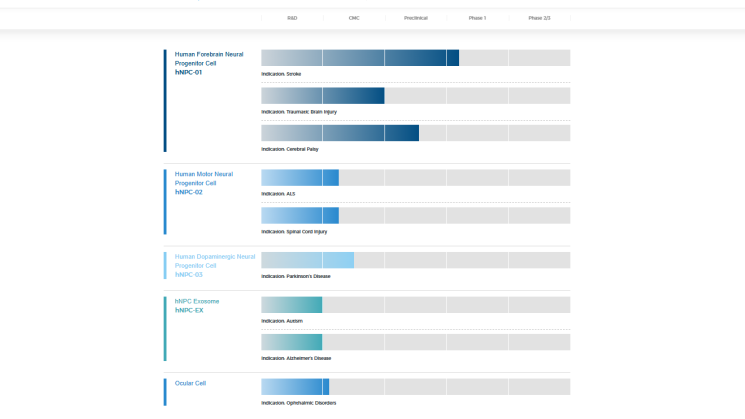
In March this year, the company announced that hNPC01 had been approved by the US FDA IND.
Wuhan Ruijian Pharmaceutical Technology Co., Ltd.
Ruijian Pharmaceutical is an international innovative biomedical company focusing on the field of regenerative medicine. Through a unique technology platform, it focuses on small molecule chemical transcription regulation of iPSC. Currently, NouvNeu001 is in clinical phase 1/2 for the treatment of Parkinson's disease (the world's first chemical induction-based universal cell therapy product to enter the clinical phase), and NouvNeu003 is in clinical phase 1 for the treatment of early-onset Parkinson's disease.
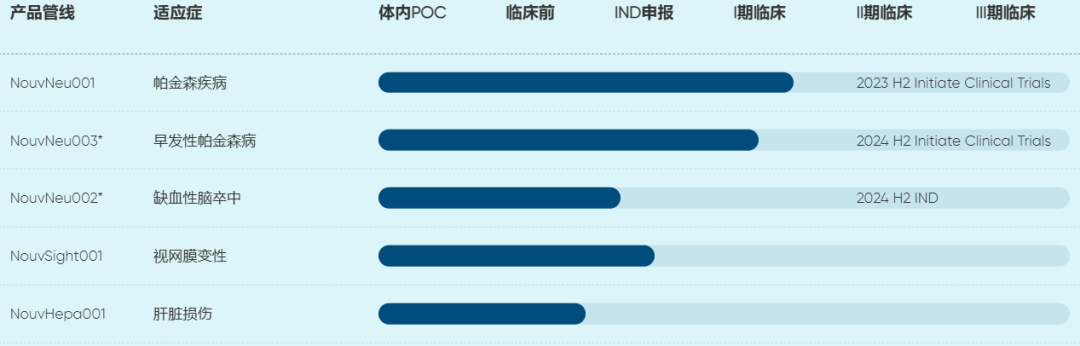
In March this year, NouvNeu001 was granted a special exemption by the U.S. FDA and was approved by the FDA IND in June.
In October this year, the company announced that it had completed more than 100 million yuan in Series B financing. The funds will be used to strengthen the R & D promotion of the company's various innovation pipelines and build clinical capabilities, as well as increase the company's industrialization efforts.
Beijing Chengnuo Medical Technology Co., Ltd.
Chengnuo Medicine is jointly founded by scientists from China and the UK. It focuses on the development and application of cell and gene therapy drugs with "reprogramming technology" as its core. Currently, ALF201 (iPSC-derived endothelial progenitor cells) for the treatment of acute ischemic stroke and ALF202 (iPSC-derived endothelial progenitor cells) for the treatment of severe lower limb ischemia are in clinical stage 1.
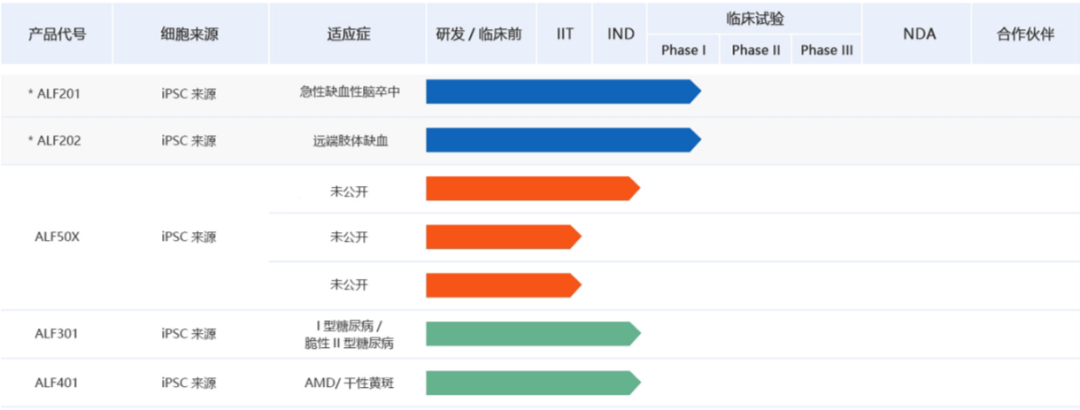
In September this year, the company announced that ALF202 had successfully completed the enrollment and administration of all subjects in the multi-center Phase 1 clinical trial.
Hangzhou Qihan Biotech Co., Ltd.
Qihan Bio is a biotechnology company that applies high-throughput gene editing technology to the fields of cell therapy and organ transplantation. Based on its high-throughput gene editing technology and a deep understanding of immune transplantation knowledge, it develops immunologically compatible allogeneic cell therapy and xenogeneic organ therapy. QN-019a (iPSC-derived CAR-NK) is currently in clinical stage 1 for the treatment of CD19-positive relapsed and refractory aggressive B-cell non-Hodgkin lymphoma.
In April this year, the company published its latest progress in xenotransplantation in Microbiology Spectrum-multi-gene editing through the CRISPR-Cas system to help pigs resist African swine fever virus.
Help Therapeutics Co., Ltd.
Help Therapeutics is a leading global innovative cardiovascular therapy development company. It has created Help Cell-foundation, an intelligent platform for three major systems, to continuously promote the development of innovative treatment products around degenerative diseases such as the cardiovascular system. HiCM-188 (iPSC-derived cardiomyocytes) is currently in clinical stage 1 for the treatment of severe congestive heart failure.
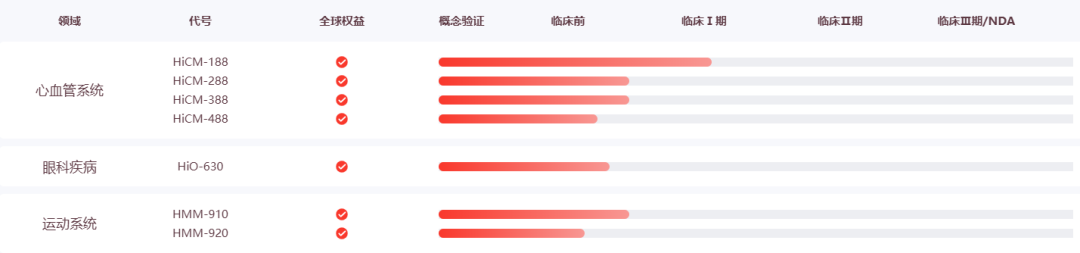
In October this year, the company announced that HiCM-188 was officially approved by the US FDA IND.
In November this year, the company's ongoing clinical trial of HiCM-188 in the treatment of severe ischemic heart failure was included in the international authoritative medical journal "Nature Reviews Cardiology".
Hangzhou Reprogenix Bioscience,Inc.
Reprogenix Bio is a disruptive regenerative medicine technology company that takes stem cell treatment of diabetes as an entry point and is committed to expanding the application of cell therapy in various disease fields. In October this year, its product RGB5088 (autologous iPSC-derived islet cells) was approved for clinical treatment of diabetes (the first approved pancreatic cell product derived from human pluripotent stem cells in China).
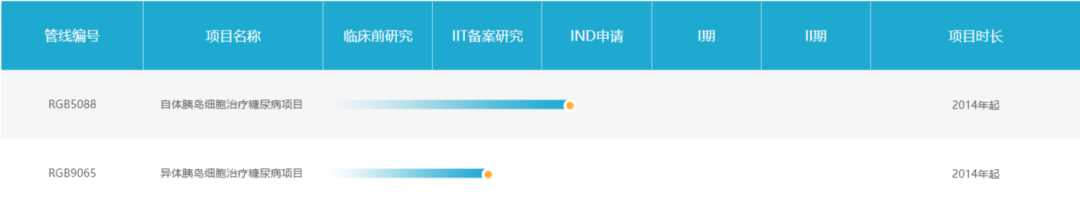
In November this year, the company announced that it had completed Series A financing of more than 100 million yuan. The capital market once again attracted widespread attention on the layout of diabetic stem cell therapy.
Suzhou Fuent Pharmaceutical Co., Ltd.
Fuente is committed to comprehensive deployment in fields such as anti-tumor drugs through its independently developed patented breakthrough protein transport technology and patented technology for in situ cell reprogramming of disease tissues in vivo. This year, SON-DP has been approved for clinical treatment of advanced malignant solid tumors.
Shize Biomedical (Suzhou) Co., Ltd.
Shize Bio is committed to providing large-scale, low-cost stem cell treatment solutions for major diseases represented by Parkinson's disease that have no substantial clinical solutions.
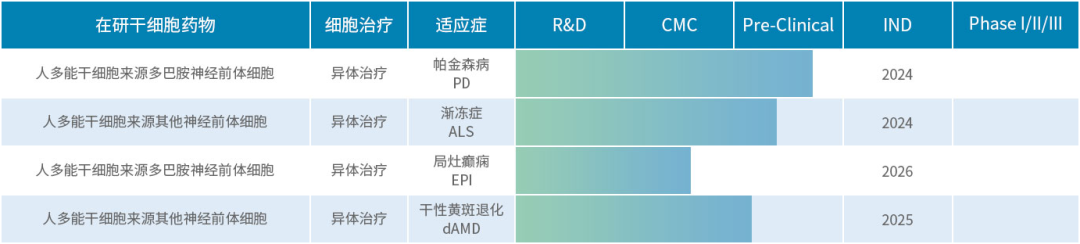
In October this year, the company announced that it had completed a B1 round of market-oriented financing of more than 100 million yuan, which will be used to improve the pipeline of clinical iPSC-derived cell drugs for the treatment of nervous system diseases, further carry out clinical research, and promote multiple registered clinical trials.
In November this year, the company teamed up with Liu Zhongmin's team from Shanghai City Oriental Hospital (Oriental Hospital Affiliated to Tongji University) and others to successfully complete the enrollment of the first patient in "clinical grade iPSC-derived subtype neural precursor cells for the treatment of ALS."
Shanghai Yuesai Biotechnology Co., Ltd.
Yuesai Bio is committed to developing a new generation of cell therapy drugs based on human pluripotent stem cell technology, and its research pipeline covers Parkinson's disease and other neurodegenerative diseases. Recently, its product UX-DA001 (autologous iPSC-derived midbrain dopaminergic nerve precursor cells) was approved for clinical treatment of primary Parkinson's disease.
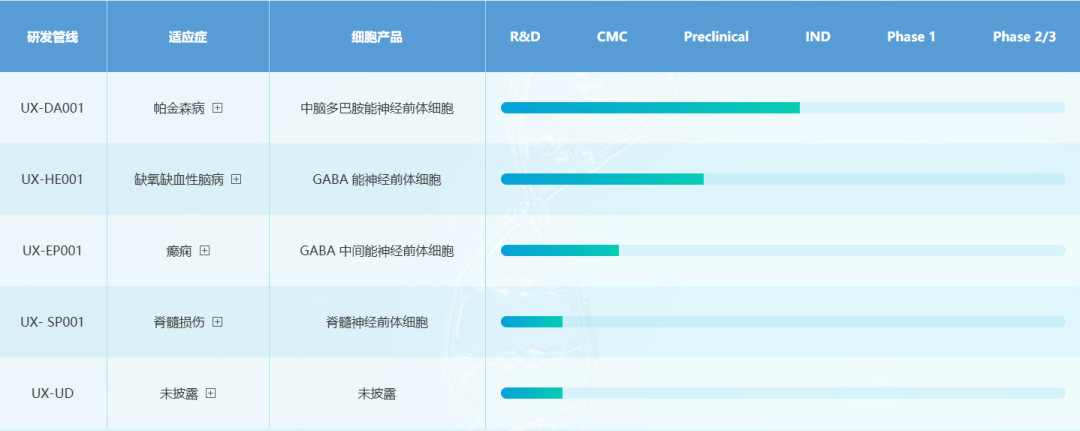
In April and July this year, the company completed more than 100 million yuan in Series A financing and tens of millions of Series A+ financing respectively. The funds will be mainly used to promote the company's innovation pipeline in the field of stem cell therapy and accelerate clinical transformation.
3 summary
At present, the global iPSC technology development momentum is becoming increasingly strong. Domestic iPSC companies continue to advance clinical research this year, laying a solid foundation for the long-term development of the industry. At the same time, many companies are bucking the current in the capital market and successfully obtaining a new round of capital injections, reflecting investors 'firm optimism about the prospects of iPSC technology. In the future, we hope that iPSC technology can be applied in more diseases and promote the continuous advancement of cell therapy.