The latest breakthrough in global pluripotent stem cell therapy in 2025:115 clinical trials reveal the future medical revolution
Researchers from the Novo Nordisk Foundation Center for Stem Cell Medicine (reNEW) and the Department of Neuroscience at the School of Health and Medical Sciences at the University of Copenhagen published a review article on Cell Stem Cell on January 2, 2025."2025 Clinical Trial Update of Pluripotent Stem Cell Derived Therapies" It reviews the progress of clinical applications of pluripotent stem cell (hPSC) derived therapies around the world.
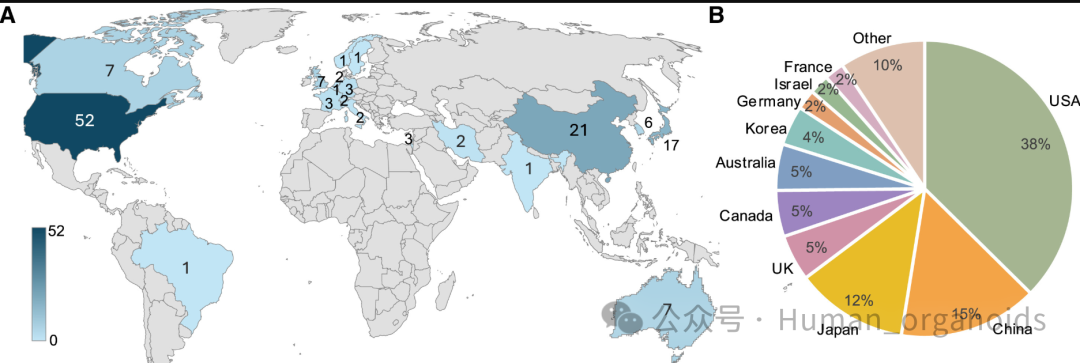
Since the first isolation of human pluripotent stem cells (hPSCs), major breakthroughs have been made in cell differentiation and production technology. Currently, there have been 115 clinical trials with regulatory approval, involving 83 hPSC-derived products, covering eye diseases, central nervous system diseases and other fields. By the end of 2024, more than 1200 patients have been treated, with a total of more than 10 ‰ cells transplanted, and no extensive safety issues have been found.
This article reviews the development of hPSC therapy, covering the application of embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs). The discovery of hESCs has enabled researchers to prepare specific types of human cells on a large scale, and the birth of hiPSCs has further improved the feasibility of clinical applications and reduced the risk of immune rejection. However, the clinical transformation of hPSC therapy still faces challenges, including issues such as cell differentiation purity control, animal model construction, safety assessment and production standardization.
Major clinical trials and related companies
1. Eye diseases (Ophthalmic Disorders)
hPSC-derived retinal pigment epithelium (RPE) cells are one of the first products to enter clinical trials and are mainly used to treat age-related macular degeneration (AMD), Stargardt's disease (SMD) and retinitis pigmentosa (RP).
-
- MA09-hRPE (hESC-derived RPE cell suspension) developed by Astellas (formerly Advanced Cell Technology) has been tested in the United States, the United Kingdom and South Korea (NCT01344993, NCT01674829, etc.) to treat dry AMD and SMD. Preliminary data show that the vision of some patients has improved, but the efficacy varies greatly from individual to individual.
- The hiPSC-RPE trial (UMIN000011929) led by Masayo Takahashi's team at the RIKEN Institute (Japan) is the world's first clinical application of iPSC-derived cells. The study used autologous iPSC-RPE cell slices to treat patients with wet AMD and showed signs of graft survival without immunosuppression.
- OpRegen (RG6501)(hESC-RPE cell suspension) developed by Lineage Cell Therapeutics (formerly BioTime) is used to treat dry AMD and will enter a Phase II clinical trial in 2023 (NCT05626114).
In addition, it also includes:
- PF-05206388 (hESC-RPE monolayers) for wet AMD was conducted at Moorfields Eye Hospital in the UK (NCT01691261).
- iPSC-RPE/PLGA (hiPSC-derived RPE cells containing PLGA scaffolds) developed by NIH/NEI (National Institute of Ophthalmology) entered Phase I/II clinical trials in 2020 (NCT04339764).
2. Central nervous system diseases (CNS Disorders)
Neural cell products derived from hPSC are mainly used to treat Parkinson's disease (PD), spinal cord injury (SCI), stroke and epilepsy.
-
- Bemdaneprocel (hESC-derived dopamine neural progenitor cells) developed by BlueRock Therapeutics is used to treat Parkinson's disease and has entered Phase I clinical trials in the United States and Canada (NCT04802733).
- iPSC-DA progenitors (hiPSC-derived dopamine neural progenitor cells) led by Jun Takahashi at Kyoto University's CiRA (iPSC Research Center) have completed a Phase I/II trial in Japan (UMIN000033564), and some patients have improved motor function.
- GRN-OPC1 (hESC-derived oligodendrocyte progenitor cells) developed by Lineage Cell Therapeutics (formerly Asterias Biootherapeutics) is used to treat spinal cord injury. A Phase I trial (NCT01217008) has been carried out in the United States, but it has been suspended due to financial reasons and is currently being promoted by a new company.
- NRTX-1001 (hESC-derived inhibitory interneuron) developed by Neurona Therapeutics for the treatment of refractory temporal lobe epilepsy has entered Phase I/II trials in the United States (NCT05135091).
3. Endocrine and metabolic disorders
hPSC-derived islet cell products are mainly used to treat type 1 diabetes (T1DM).
-
- VX-880 (hESC-derived islet cells) developed by Vertex Pharmaceuticals is used to treat type 1 diabetes, where patients do not require exogenous insulin supplementation after receiving transplantation (NCT04786262).
- ViaCyte (later acquired by Vertex) developed VC-01 and VC-02 (hESC-derived pancreatic endodermal cells), which were encapsulated in Encaptra devices, respectively, to improve cell survival (NCT03163511, NCT02239354).
- Tianjin First Center Hospital conducted a phase 1 clinical trial based on chemical reprogramming of iPSC-derived islet cells (ChiCTR2300072200).
4. Cancer and Immunotherapy
hPSC-derived immune cell therapy focuses mainly on natural killer (NK) cells and T cell therapies.
- Fate Therapeutics has developed a variety of hiPSC-based NK cell therapies:
-
- FT500 (NCT03841110): Used for the treatment of advanced solid tumors.
- FT516 (NCT04023071): Targeting B-cell lymphoma.
- FT596 (NCT04245722): Contains CD19-CAR and is used for B-cell malignancies.
- FT538 (NCT04614636): For use in acute myeloid leukemia (AML) and multiple myeloma (MM).
-
- CNTY-101 (CD19-CAR NK cells) developed by Century Therapeutics has entered Phase I clinical practice (NCT05336409).
- MEG-002 (hPSC-derived platelets) was developed by Megakaryon Corporation (Japan) for the treatment of thrombocytopenia (JRCT2053210068).
Future prospects
At present, clinical trials of pluripotent stem cell-derived therapies are still mainly Phase I/II, with the main goal of assessing safety and feasibility, and a few trials have begun to enter the efficacy evaluation stage. Future development directions include:
- Optimize immune compatibility: Reduce immune rejection through HLA matching strategies and improve long-term survival of transplanted cells.
- Improve cell survival and function: Improve the maturity and stability of stem cell-derived cells, such as applying gene editing techniques to improve the function of islet cells and nerve cells.
- Reduce production costs: Reduce the cost of stem cell therapies through large-scale automated production and make them more accessible.
This review provides a comprehensive perspective on the latest clinical progress of pluripotent stem cell therapy and looks forward to its application potential in future medicine.
References:Pluripotent stem-cell-derived therapies in clinical trial: A 2025 update.Kirkeby, Agnete et al. Cell Stem Cell, Volume 32, Issue 1, 10 - 37

