
On January 2, 2025, China's State Food and Drug Administration (NMPA) officially approved the marketing of Amimetoxin injection, marking the advent of my country's first mesenchymal stem cell (MSC) treatment. The drug is mainly used to treat acute graft-versus-host disease (aGVHD) in those over the age of 14 who have failed hormone therapy, providing new treatment options for patients in this field.
Aimimaitoxin Injection is a human umbilical cord mesenchymal stem cell injection developed by Platinum Excellence Biotechnology (Beijing) Co., Ltd. Since the first submission of a new drug clinical study application in 2013, this product has gone through years of research and development and clinical trials. It has shown certain therapeutic effects in phase II clinical trials, especially in patients who have received multiple infusions.
Next, let's take stock of the 16 known mesenchymal stem cell drugs on the market around the world.
Among them, 10 are derived from bone marrow, 3 are derived from umbilical cord, 2 are derived from fat, and 1 is derived from cord blood.
In terms of sales, Temcell, which treats graft-versus-host disease, and Cartistem, which treats degenerative arthritis, perform better in the market.
Mesoblast products
The research and development process from Prochymal to Ryoncil went through the United States, Canada, Australia and Japan, with an interval of 12 years, and finally became the first mesenchymal stem cell drug approved by the FDA.
Mesenchymal stem cell drugs ①
Prochymal: Graft-versus-host disease
Ratification country: Canada
Date of approval: May 17, 2012
Cell type: Bone marrow mesenchymal stem cells
Indication: Childhood acute graft-versus-host disease (GvHD)
Price: US$200,000/course (usually 4 doses)
Sales: Unknown
Overview: Prochymal is a ready-to-use stem cell product that can be infused via simple intravenous injection without the need for matching or immunosuppression. Available through extended access procedures in the United States, and has also been approved in countries such as New Zealand and Switzerland.
Prochymal was first approved in the United States in 2009 and was subsequently sold by Osiris to Mesoblast in Australia and renamed Ryoncil.

Mesenchymal stem cell drugs ②
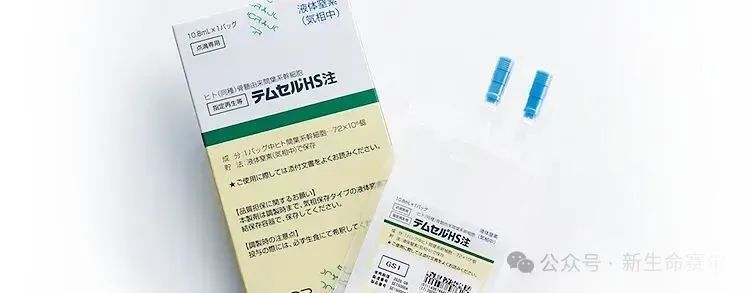
Temcell: Graft-versus-host disease
Ratification country: Japan
Date of approval: September 2015
Cell type: Bone marrow mesenchymal stem cells
Indication: Acute graft-versus-host disease (GvHD)
Price: 868,680 yen (approximately US$7,700) per bag, a standard course of treatment usually requires 8 bags, so the total cost is approximately US$123,000) to US$185,000.
Sales: The financial report on October 30, 2024 showed that sales for the quarter were 1.5 billion yen (approximately US$9.5 million)
Overview: Temcell was developed by Mesoblast and sold by JCR Pharmaceuticals in Japan. Temcell has shown good efficacy in clinical trials, especially in the treatment of steroid-refractory acute graft-versus-host disease. Based on clinical data, the overall response rate after 28 days for patients treated with Temcell reached 56%.
In addition, Temcell is also included in Japan's National Health Insurance System, which allows patients to enjoy partial reimbursement of expenses during treatment, thereby reducing the actual payment burden.
Mesenchymal stem cell drugs ③
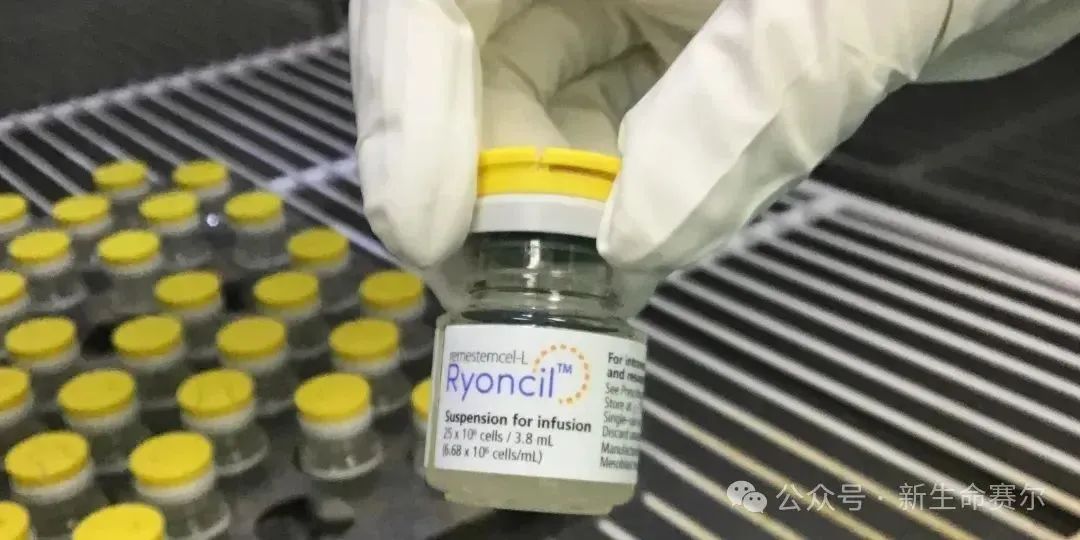
Ryoncil: Graft-versus-host disease
Ratification country: United States
Approval date: December 18, 2024
Cell type: Bone marrow mesenchymal stem cells
Indication: For the treatment of steroid-refractory acute graft-versus-host disease (SR-aGVHD) in children 2 months and older.
Price: Not yet disclosed
Overview: This is the first mesenchymal stem cell (MSC) therapy approved by the FDA. In a multicenter, single-arm phase III clinical trial, 70% of patients showed an overall response after 28 days of treatment with Ryoncil, a measure that correlates with survival.
Ryoncil, an innovative mesenchymal stem cell treatment product, provides new treatment options for patients with steroid-refractory acute graft-versus-host disease and is expected to expand to other indications in the future.
Korean products
As one of the countries with the largest number of mesenchymal stem cell drugs, South Korea has not approved new products in the past 10 years, and the market performance of existing products is also very different.
Mesenchymal stem cell drugs ④
CellGram: Acute myocardial infarction
Ratification country: South Korea
Date of approval: July 1, 2011
Cell type: Autologous bone marrow mesenchymal stem cells
Indication: Acute myocardial infarction (AMI)
Price: 18 million won (approximately US$15,000) and is not covered by medical insurance reimbursement.
Sales: In 2018, it peaked at 2.5 billion won and then began to decline, reaching 800 million won in 2023.
Overview: CellGram is considered to be the world's first approved stem cell drug, marking an important milestone in the field of stem cell therapy. In clinical trials, patients treated with CellGram showed an improvement in left ventricular ejection fraction of approximately 6% after 6 months.
CellGram needs to provide clinical data on 600 patients within 6 years when it comes to market. Pharmicell later applied to reduce the number to 60 but was rejected. This situation has drawn industry attention to its future approval status. In 2022, CellGram was re-evaluated as an advanced biopharmaceutical product, indicating that it still has certain development potential under regulatory policies.

Mesenchymal stem cell drugs ④
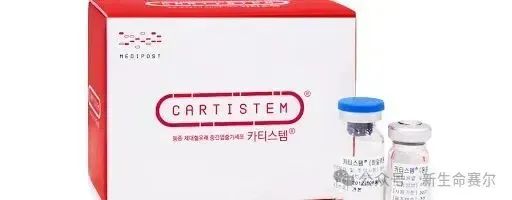
Cartistem: Degenerative arthritis
Ratification country: South Korea
Date of approval: January 2012
Cell type: cord blood mesenchymal stem cells
Indication: Degenerative arthritis and knee cartilage damage
Price: $19,000-$21,000
Sales: The cumulative number of patients administered to drugs exceeded 31,000, and cumulative sales reached 108 billion won, exceeding 20 billion won (approximately US$14.4 million) in 2023.
Overview: Cartistem is the world's first allogeneic cord blood stem cell therapy that has proven effective in suppressing inflammation and regenerating damaged cartilage tissue.
Medipost is preparing for Phase 3 clinical trials of Cartistem in Japan and the United States, and also plans to enter ASEAN countries such as Malaysia. In Japan, if conditions are met, Phase 1 and 2 clinical trials can be skipped and Phase 3 can be directly entered.
Mesenchymal stem cell drugs ④
Cupistem: Crohn's disease
Ratification country: South Korea
Date of approval: January 2012
Cell type: Autologous adipose mesenchymal stem cells
Indication: Complex anal fistula in Crohn's disease
Price: About 6 million won (about US$5000/time)
Sales: Undisclosed
Overview: A distinctive feature of Cupistem is its sustainability of efficacy. Among the patients treated, 81% remained healed after 96 weeks. Cupistem received medical insurance price approval from the Korea Health Insurance Review and Evaluation Service (HIRA) in 2014, making it more financially possible for patients to receive the treatment.

Mesenchymal stem cell drugs ④
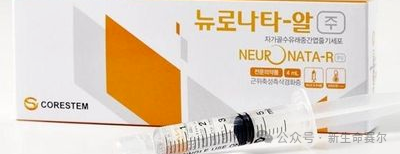
NeuroNATA-R: Amyotrophic lateral sclerosis
Ratification country: South Korea
Date of approval: July 30, 2014
Cell type: Autologous bone marrow mesenchymal stem cells
Indication: Amyotrophic lateral sclerosis (ALS) and other motor neuron diseases
Price: $18,000 to $72,000
Sales: Undisclosed
Overview: NeuroNATA-R has neuroprotective effects by injecting autologous bone marrow mesenchymal stem cells, which can delay the death of motor neurons, thereby slowing the progression of ALS. In the Phase I clinical trial to assess safety, a total of 7 patients received intrathecal injection and were followed up for 12 months. The results showed that this treatment regimen has good safety.
Indian products
Mesenchymal stem cell drugs ⑧
Stempeucel: Severe limb ischemia
Ratifying countries: India, EU
Date of approval: June 2015 (Obtained orphan drug status in the EU)
Cell type: Bone marrow mesenchymal stem cells
Indication: Severe limb ischemia
Price: US$2200 (2017)
Sales: Undisclosed
Overview: Stempeucel has been developed for 12 years and uses unique pooling technology to make the production process more efficient and provide treatment to a large number of patients. Clinical trials have shown that Stempeucel has significant effects in improving blood flow and function in patients with severe limb ischemia.
Japanese products
Mesenchymal stem cell drugs ⑨
Alofisel: Crohn's disease
Ratifying countries: EU, Japan
Date of approval: March 23, 2018 (approved in the European Union), and subsequently approved in Japan.
Cell type: Adipose mesenchymal stem cells
Indication: Complex anal fistula
Price: 5.62 million yen/dose (approximately US$36,000)
Sales: In fiscal 2020, Takeda generated approximately $350,000 in revenue from Alofisel.
Overview: Alofisel has shown good safety in clinical trials, but a recent study showed that its effectiveness is comparable to placebo, so its clinical benefits are in question. In December 2024, the European Union decided to withdraw Alofisel's marketing authorization due to failure to provide sufficient data to confirm its efficacy.

Mesenchymal Stem Cell Drugs ⑩
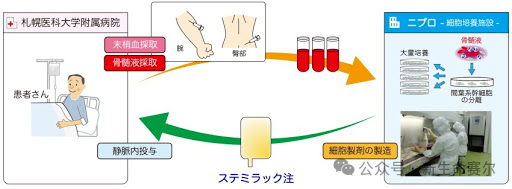
Stemirac: Spinal cord injury
Ratification country: Japan
Date of approval: November 21, 2018 (conditional and time-limited approval was obtained)
Cell type: Bone marrow mesenchymal stem cells
Indication: Neurological dysfunction associated with spinal cord injury
Price: About 15 million yen/dose (US$95,000)
Sales: A 2019 article predicted that Stemirac will generate approximately US$3.4 million in annual revenue to Nipro over the next nine years.
Overview: Stemirac received conditional and time-limited approval in Japan to allow it to be sold on the market, while requiring Nipro to provide further clinical data within seven years to demonstrate its effectiveness. Stemirac has shown good safety in clinical trials and has been recognized by the Japanese government as a breakthrough product aimed at accelerating its marketing process to provide new options for patients in urgent need of treatment.
Mesenchymal stem cell drugs ⑪
AKUUGO: Traumatic brain injury
Ratification country: Japan
Approval date: July 31, 2024 (conditional and time-limited approval is obtained)
Cell type: Bone marrow mesenchymal stem cells
Indication: Improvement of chronic sports paralysis caused by traumatic brain injury (TBI)
Price: Not yet disclosed
Sales: Not yet disclosed
Overview: AKUUGO is an allogeneic mesenchymal stem cell product. It enhances the ability to regenerate nerve cells by temporarily transfecting the human Notch-1 intracellular domain gene into cultured cells. The research results show that AKUUGO not only has neuroprotective effects, but also promotes angiogenesis and plays an immunoregulatory function.
Iranian products
Mesenchymal stem cell drugs
Mesestrocell: Osteoarthritis
Ratification country: Iran
Date of approval: 2018
Cell type: Bone marrow mesenchymal stem cells
Indication: osteoarthritis
Price: 200 million Iranian rials (approximately US$4600)
Mesenchymal stem cell drugs ⑬
StemSin
Ratification country: Iran
Date of approval: 2018
Cell type: Bone marrow mesenchymal stem cells
Indication: Multiple sclerosis
Price: Undisclosed
Mesenchymal stem cell drugs ⑭
AlloStemSin
Ratification country: Iran
Date of approval: 2018
Cell type: Umbilical cord mesenchymal stem cells
Indication: Amyotrophic lateral sclerosis
Price: Undisclosed
Mesenchymal Stem Cell Drugs ⑮
Vartocell
Approved country: Iran
Approval date: 2020
Cell type: Umbilical cord mesenchymal stem cells
Indication: Cerebral palsy
Price: 100 million Iranian rials (each bottle contains 20 million cells), approximately 2,300 US dollars
China products

Mesenchymal Stem Cell Drugs ®
Aimitosa Injection: Graft-versus-host disease
Approved country: China
Approval date: January 2, 2025
Cell type: Umbilical cord mesenchymal stem cells
Indication: For acute graft-versus-host disease (aGVHD) in patients over the age of 14 who have failed hormone therapy, especially in cases with dominant digestive tract involvement.
Price: Not yet disclosed
Overview: This is the first approved stem cell therapeutic drug in China, marking an important milestone in the research and development of domestic stem cell drugs. Amimetoxin injection has shown significant efficacy and safety in clinical trials by using human umbilical cord mesenchymal stem cells to repair damaged tissue and regulate immune function.
The drug was marketed through the priority review and approval process of the State Food and Drug Administration of China, which means that it has obvious clinical needs and potential advantages. It will be used as a prescription drug in hospitals, providing patients with new treatment options.
Abandon selection
MPC:
Mesoblast's independently developed mesenchymal stem cell products have not yet been formally approved.
Queencell:
According to the "Korean Regenerative Medicine Business Development and Industry" report released by the Korea Pharmaceutical and Biopharmaceutical Manufacturers Association (KPBMA) in 2019, Queencell is defined as a minimally processed cell product.
Holociar:
Developed by Casey Pharmaceuticals of Italy and approved for the treatment of limbal stem cell deficiency (LSCD). The active ingredient is autologous limbal stem cells.