
According to a newly published review by Professor Wang Renzhi's team from the Department of Neurosurgery at Peking Union Medical College Hospital, stem cell therapy is reshaping the treatment landscape of nervous system diseases.
In order to comprehensively assess the development status of this field, based on the Trialtrove database, a systematic analysis was conducted on 530 relevant clinical trials conducted around the world as of the end of 2023.
The article discusses it from the dimensions of time evolution, regional distribution, research hotspots and future trends, providing reference for academia and industry.
Research overview and methods
By integrating Trialtrove, ClinicalTrials.gov and EMA databases, the author adopted a multi-dimensional search strategy (keywords: stem cell/cell therapy; treatment field: central nervous system) and finally included 530 clinical trials (including 2 cross-validated supplementary documents) after manual screening.
Analysis of study status showed that 299 trials (56.4%) had been completed, of which 24.1% had achieved positive results; 127 were ongoing studies (24.0%); and 65 were terminated (12.3%), mainly due to factors such as difficulty in enrollment, lack of funds and insufficient efficacy.
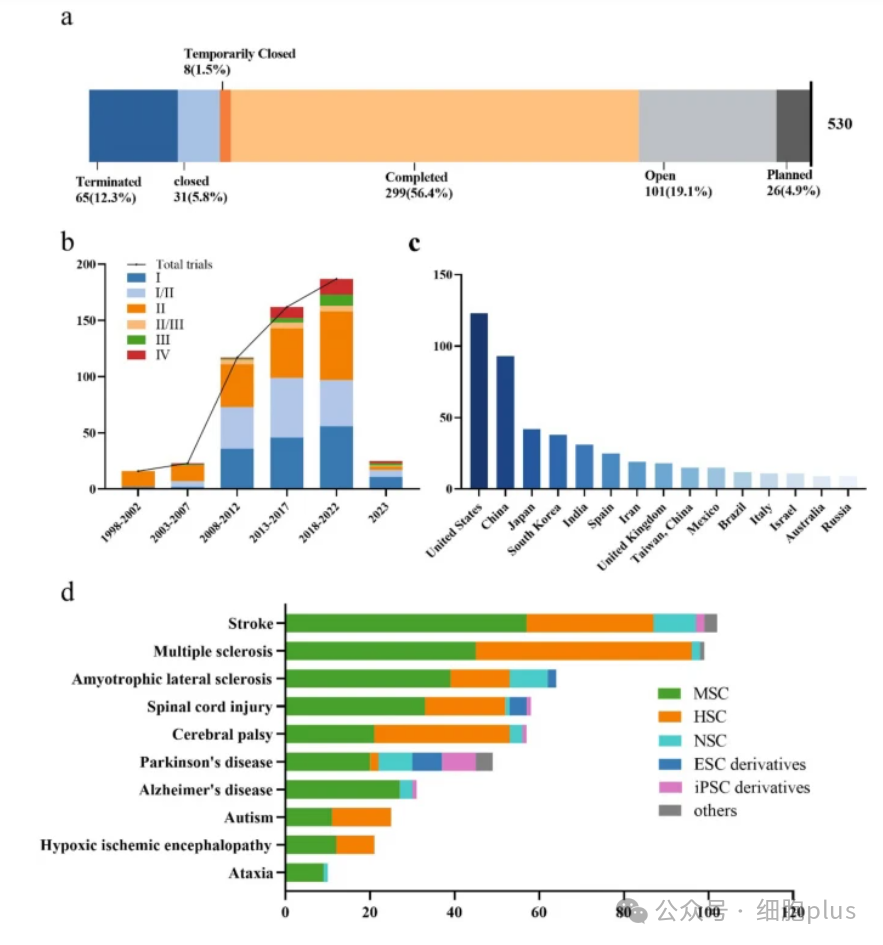
A global overview of stem cell therapy trials for nervous system diseases.
a: Stem cell therapy trials, by status, as of December 31, 2023.
b: The number of global clinical trials from 1998 to 2023, divided into five years, and an independent analysis will be conducted in 2023. Each period includes the total number of trials and a breakdown by phase.
c: Number of clinical trials in the top 15 countries and regions.
d: Top 10 neurological diseases treated with different stem cell types.
Time evolution and stage characteristics
Since the first trial was launched in 1998, the number of studies has shown a step-by-step growth: it accelerated significantly from 2008 to 2012 and peaked from 2018 to 2022 (Figure 1b).
The main research phases are Phase II (32.8%) and Phase I/II (26.8%), and Phase III/IV only accounts for 6.8%, reflecting that this field is still in the early stages of clinical exploration.
In terms of funding, academia led 72.6% of research, industry participation was 33.2%, and government funding accounted for 10.0%(Table S1).
This structural feature suggests that accelerating the transformation of results requires strengthening multi-party collaboration, especially innovative support from regulatory policies.
Geographical distribution and global pattern
The United States leads the world with 123 trials (23.2%), China (93 trials, 17.5%) has become the main research force in Asia, and Spain (25 trials) represents the highest activity in Europe (Figure 1c).
It is worth noting that only Egypt in the African region carries out relevant research, highlighting the imbalance in the allocation of scientific research resources between regions. This difference is not only limited by infrastructure and financial support, but also closely related to the perfection of the regulatory system.
Establish international cooperation mechanisms, promote technology transfer, or an effective way to narrow regional gaps.
Disease lineages and cell types
Stroke (18.9%), multiple sclerosis (18.3%), ALS (11.3%) and spinal cord injury (10.2%) constitute the main indications (Figure 1d). Cell therapy strategies are diversified:
Mesenchymal stem cells (MSC): dominant (51.7%), with advantages due to ease of availability and immunoregulatory properties
Neural stem cells (NSC): Italian team makes breakthrough progress
Pluripotent stem cells: ESC-derived therapies are emerging in the treatment of Parkinson's disease, such as the STEM-PD trial (NCT05635409) and the BRT-DA01 study (NCT04802733)
iPSC: Japan and the United States take the lead in exploring personalized treatment, which has both ethical advantages and efficacy potential
challenges and prospects
Despite the surge in research, three major bottlenecks still need to be addressed:
Lack of late clinical evidence: less than 7% of trials above phase III
Transformation efficiency needs to be improved: industry participation is lower than basic research
Imbalanced regional development: Participation in Africa and other regions needs to be improved urgently
Future breakthrough directions include: establishing a special fund pool, formulating accelerated approval paths, promoting transnational multi-center research, and conducting real-world evidence collection. Special attention needs to be paid to new technology routes such as iPSC, whose personalized treatment characteristics may reshape existing treatment paradigms.
study limitations
It should be pointed out that although data integrity is improved through multi-platform cross-verification, the regional preference of registration platforms may lead to the omission of some trials. As new technologies and new policies evolve, continuous tracking and updating of databases is crucial.
This study reveals through panoramic analysis that stem cell therapy has opened up new dimensions for the treatment of nervous system diseases, but to truly achieve clinical transformation still needs to overcome multiple barriers at the scientific, industrial and policy levels. Only through global collaborative innovation can this cutting-edge technology benefit a wider patient group.