
Source: BioValley
With the rapid development of our economy and society, coping with aging population has become a basic condition of our country. The health of the elderly population has a great impact on the family medical burden and social public health expenditure. In recent years, there has been a growing awareness of the importance of skeletal muscle for healthy aging. As one of the four major tissues of human body, muscle plays a vital role in the regulation of body movement and glucose and lipid metabolism homeostasis. Age-related declines in skeletal muscle mass and muscle strength or physical function, known as sarcopenia, not only affect patients' quality of life, but are also associated with poor prognosis in a variety of chronic diseases and malignancies. China's understanding and research on sarcopenia started late, so studying the etiology of muscle atrophy in elderly individuals and formulating effective intervention measures to prevent and control muscle loss will help to improve the quality of life of elderly individuals and accelerate the realization of "healthy aging".
Recently, A team led by researcher Liwei Xie, Institute of Microbiology, Guangdong Academy of Sciences and State Key Laboratory of Applied Microbiology in South CHINA, and Academician Yulong Yin, Institute of Subtropical Agriculture Ecology, Chinese Academy of Sciences, published a paper entitled "Dynamic Changes in SCIENCE China-Life Sciences. Butyrate Levels Regulate Satellite Cell Homeostasis by Preventing Spontaneous Activation During Aging. This study is the first to report that intestinal flora can regulate skeletal muscle satellite cell homeostasis and muscle function through butyric acid signaling pathway. The findings of this study can provide new intervention targets and early warning schemes for early prevention and intervention of skeletal muscle aging (Figure 1).

The growth, development and regeneration of skeletal muscle depend on adult stem cells - muscle satellite cells (SCs) - formed from the embryonic development mesoderm. Long-term resting satellite cells have self-renewal and high regenerative potential, ensuring that skeletal muscle maintains a viable pool of satellite cells. When skeletal muscle is subjected to external stimulation or injury, resting satellite cells can be activated, enter the cell cycle, proliferate and differentiate, fuse into muscle tubes, and then participate in muscle repair. Abnormal function of muscle satellite cells can lead to defective skeletal muscle regeneration and accelerated aging. The number of astrocytes in aged skeletal muscle is reduced, the ability to proliferate and differentiate myogenes is impaired, which leads to the defect of regeneration after muscle injury (Figure 2).
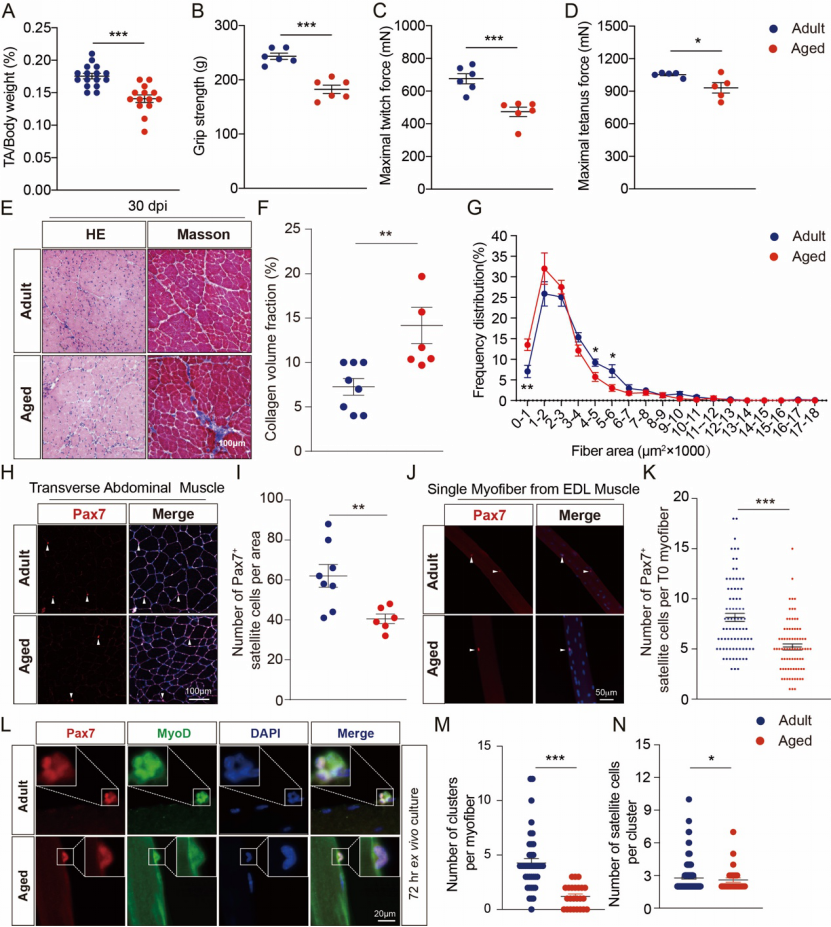
Figure 2: Aging skeletal muscle and satellite cell function decline
Intestinal flora plays an important role in various metabolic, immune and disease processes of the host. Aging is closely related to significant changes in intestinal flora. Recent studies have found that the imbalance of intestinal flora is closely related to muscle mass, function and sarcopenia. However, the role of gut flora in myosat homeostasis, especially during aging, and how it regulates myosat homeostasis and function is unclear. With the help of experimental techniques such as pseudaseptic mice constructed after antibiotic treatment and Fecal Microbiota Transplantation (FMT), this study found for the first time that intestinal flora disturbance can induce myosat cell activation (FIG. 3).
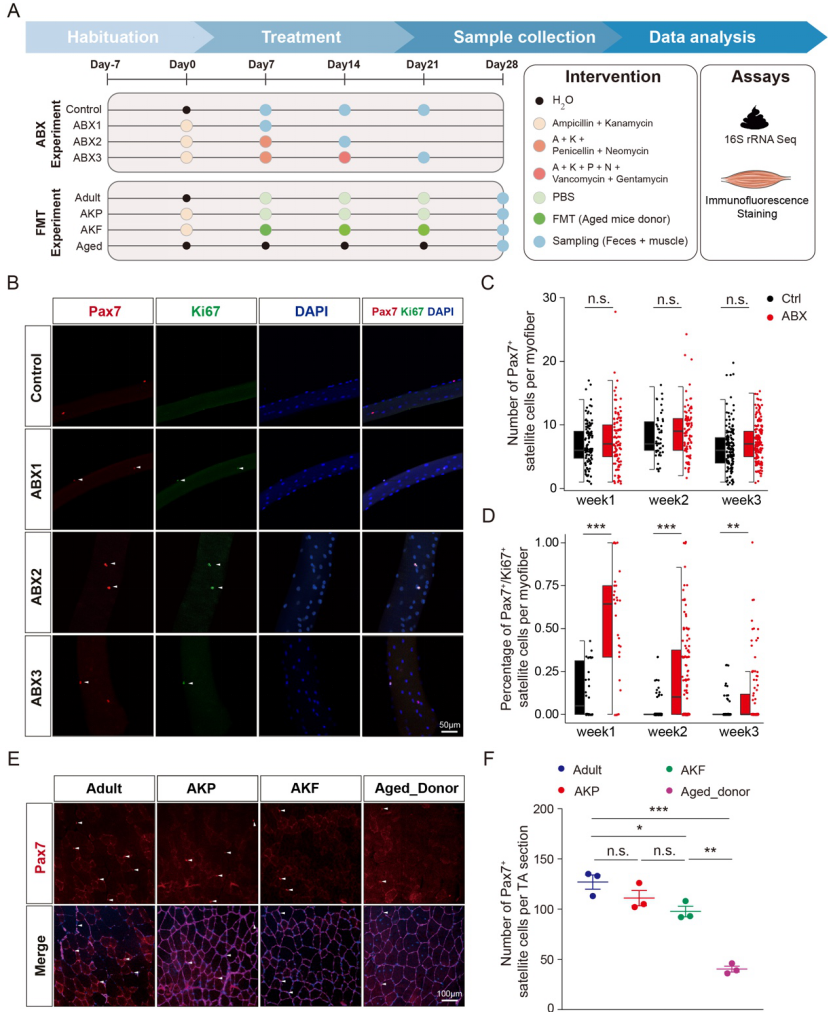
Figure 3: Changes in homeostasis of muscle satellite cells induced by changes in intestinal flora
Fecal transplantation from aged mice (80-100 weeks of age) to adult mice (10 weeks of age), accompanied by changes in the intestinal microenvironment, resulted in a decrease in the number of muscle satellite cells and muscle stretching force, as well as a decrease in skeletal muscle regeneration in young mice. These changes may be closely related to the composition and structure of intestinal microbiota and secondary metabolites from intestinal bacteria in aging mice (Figure 4).
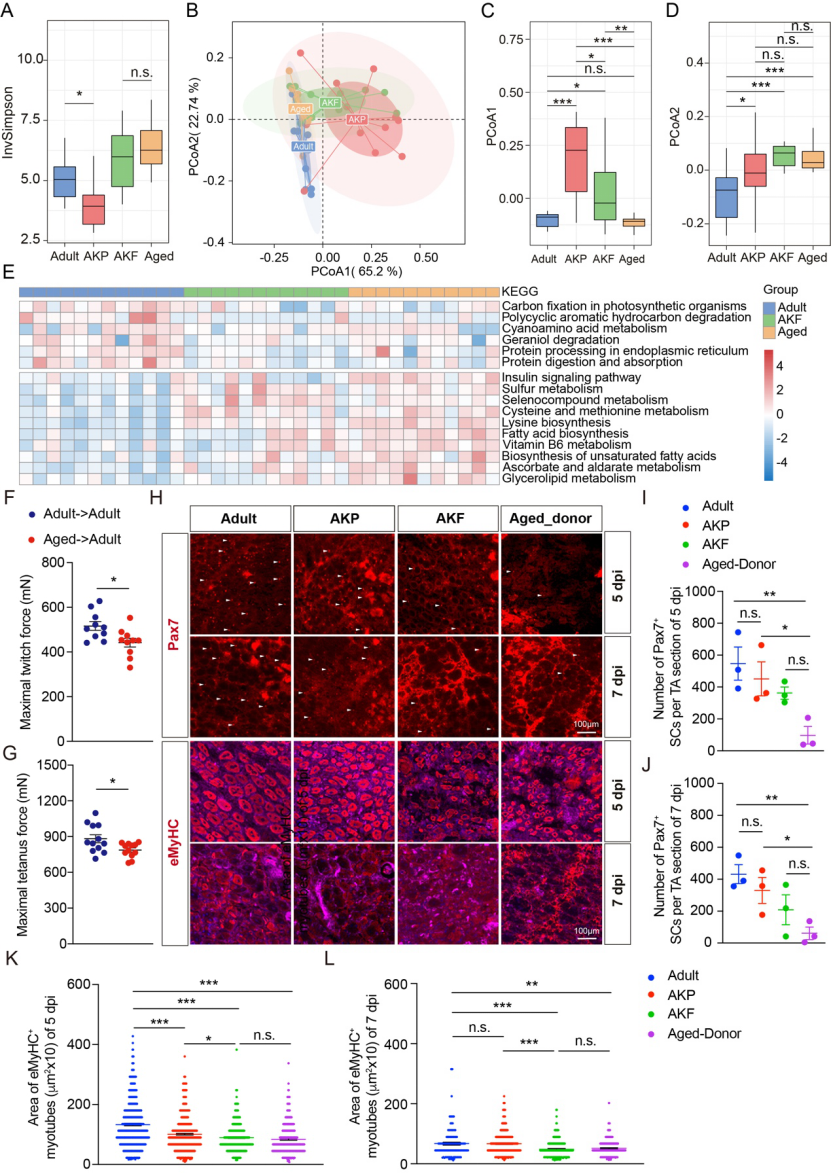
Figure 4: Dysfunction of muscle satellite cells in young mice induced by fecal bacteria transplantation in aged mice
Therefore, this study proposed a scientific hypothesis that the physiology and homeostasis of muscle satellite cells are regulated by intestinal flora and its metabolites during skeletal muscle development, aging and damage repair. To test this hypothesis, data from mice and population cohorts of different ages relied on combined analyses of multiple omics data, such as RNA-seq, 16S rRNA, and metabolomics. It has been found that with the occurrence and development of aging, the composition and structure of bacterial flora, various metabolites in feces, serum and skeletal muscle, and gene function have significant changes. Combined analysis of multiple omics data showed that butyric acid derived from intestinal flora and butyric acid metabolism may be involved in regulating the self-activation, decrease in number and dysfunction of myosatellite cells caused by aging (Figure 5).
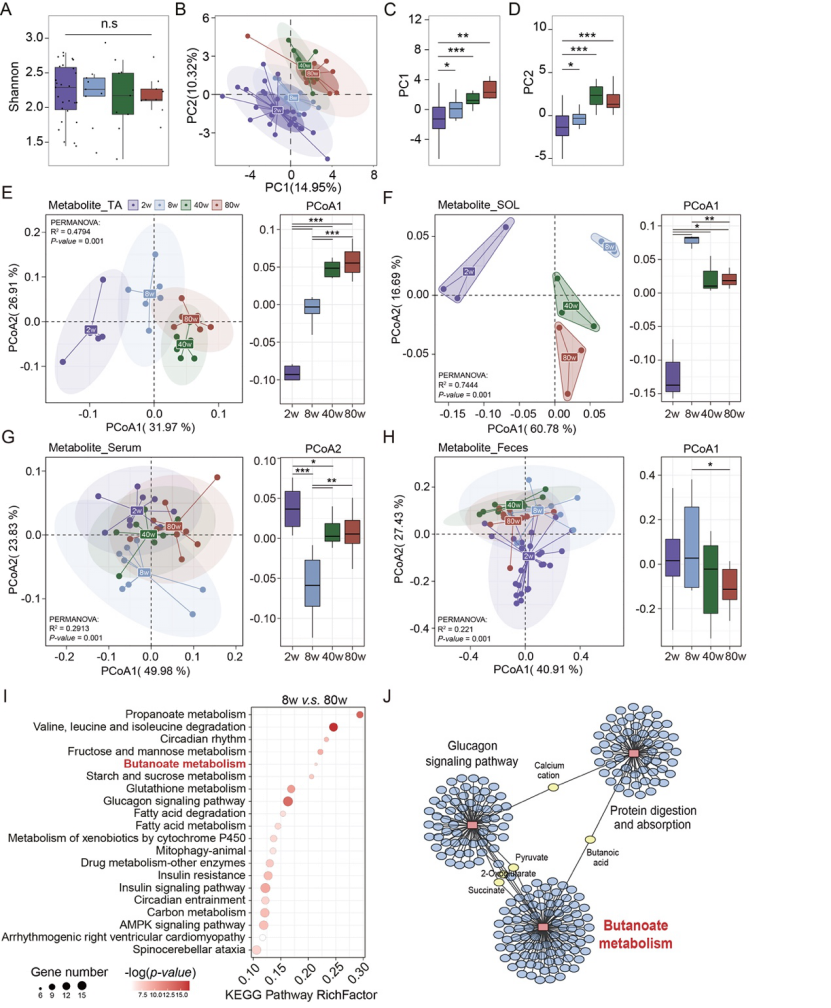
Figure 5: Dynamic changes of bacterial flora and metabolites associated with aging
The content of butyric acid in stool, serum and skeletal muscle samples of mice of different ages was analyzed by metabolomics data. It was found that butyric acid gradually decreased in serum, tibialis anterior muscle and soles muscle with aging. In addition, the antibiotic induced activation of myosatocytes in adult mice was reversed by butyric acid supplementation (Figure 6).
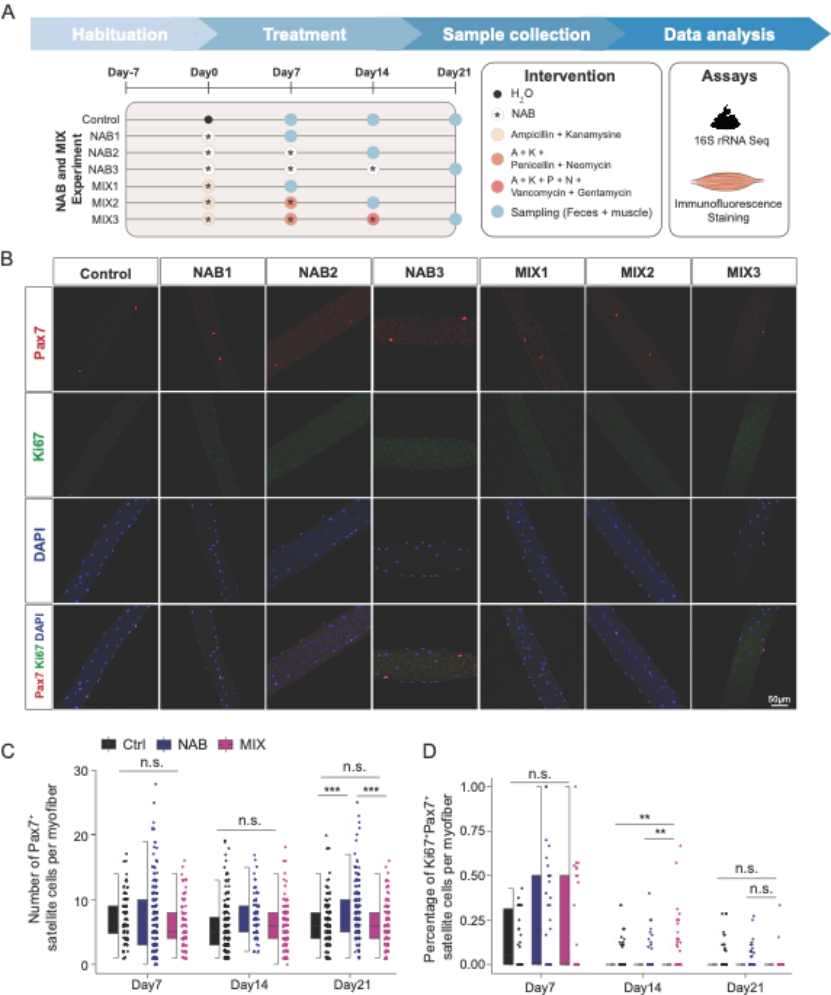
Figure 6: Skeletal muscle butyric acid reverses antibiotic induced activation of muscle satellite cells
In antibiotic treatment and butyric acid supplementation experiments, butyric acid not only inhibited antibiotic induced myosat cell activation, but also alleviated the loss of myosat cells during skeletal muscle regeneration. Therefore, butyric acid content in serum and skeletal muscle played an important role in maintaining myosat cell homeostasis. It has previously been reported that short-chain fatty acids were able to reverse muscle loss and skeletal muscle dysfunction in antibiotic or germ-free mice. In this study, we also demonstrated in vivo and in vitro that butyric acid, rather than other short-chain fatty acids (e.g., acetic acid and propionic acid), is involved in the regulation of muscle satellite homeostasis, myofibroblast proliferation and differentiation, and injury regeneration of skeletal muscle (FIG. 7).
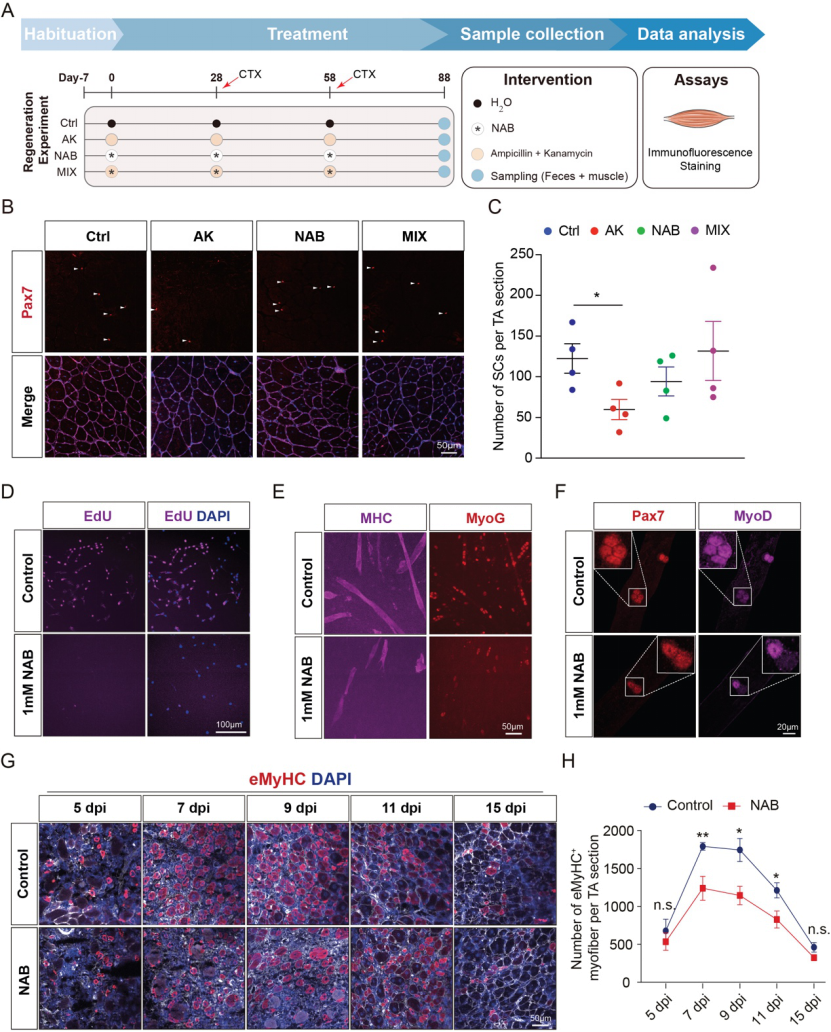
Figure 7: Butyric acid inhibits muscle satellite cell activation
Using a combination of cell and animal models, population cohort data, and multi-omics data, this study is the first to report that antibiotic-induced intestinal microbiota disturbance and the intestinal microbiota and its metabolites in aging individuals disrupt muscle satellite cell homeostasis and skeletal muscle function, in contrast to intestinal bacteria-derived metabolites. Butyric acid can alleviate the homeostasis imbalance of myosinocytes caused by antibiotic-induced intestinal flora disturbance, promote the expression of monocarboxylic acid transporter 1 (Mct1), and regulate myosinocytes homeostasis. In addition, during the regeneration process of skeletal muscle injury, the host can reduce the content of butyric acid in skeletal muscle by dynamically regulating the expression of related genes, and promote the proliferation and differentiation of muscle satellite cells and skeletal muscle regeneration. In summary, the study found that intestinal flora and its metabolites are involved in the regulation of muscle satellite cell homeostasis during aging, suggesting that the decline in butyric acid levels in muscle may be one of the causes of the loss and functional deficits of aging muscle satellite cells, which provides new targets and new ideas for the development of therapeutic strategies to prevent skeletal muscle aging and related diseases (Figure 8).
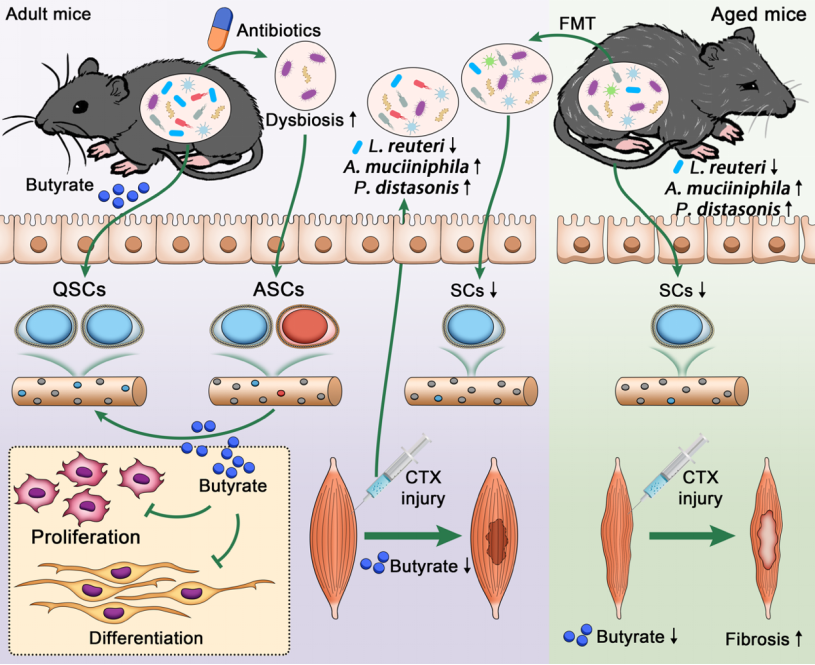
Figure: 8 Summary of relationship between aging muscle satellite cell homeostasis and intestinal flora
The corresponding author of this study is Xie Liwei, a researcher at the State Key Laboratory of Applied Microbiology in South China, Institute of Microbiology, Guangdong Academy of Sciences; the first author is Dr. Chen Shujie, a postdoctoral fellow of Xie Liwei; the co-first author is Huang Liujing, a master's candidate jointly trained by Xie Liwei and Zhujiang Hospital of Southern Medical University; and Dr. Liu Bingdong is a postdoctoral fellow of Xie Liwei. Yinyulong Academician team, the co-corresponding author of this paper, participated in the discussion of the topic.