
Source: BioArt
The plasticity of cell fate is an important scientific problem. Differentiation of somatic cells can be reversed into Induced pluripotent stem cells (iPSC) by overexpressing transcription factors or using chemical small-molecule combinations, a technique known as somatic cell reprogramming [1-13]. Compared with transcription factors, small molecules are easy to manipulate, do not insert into the genome, and are reversible. Therefore, chemical reprogramming using pure small molecule combinations is an important system for studying the plasticity of cell fate. As an emerging interdisciplinary discipline, stem cell chemical biology uses small molecules to accurately regulate the fate, state and function of stem cells in vitro and in vivo, and has wide application prospects in basic research, anti-aging, anti-tumor, regenerative medicine and other fields. Chemical reprogramming is one of its focuses. However, the reported chemical reprogramming systems lag behind the traditional transcription factor reprogramming in rate, and there is still much room for improvement. Chemical reprogramming is different from transcription factor reprogramming in molecular locus, which indicates the uniqueness of chemical reprogramming mechanism. At present, the specific molecular mechanism of chemical reprogramming process is very unclear and needs to be further explored.
On August 7, 2023, Zhu Saiyong Laboratory, Institute of Life Sciences, Zhejiang University, published A paper entitled A fast chemical reprogramming system promotes cell identity transition through Nature Cell Biology a Diapuse-like state research paper was the first to report cell Fast chemical reprogramming (FCR). In this study, the author is committed to transforming the mouse chemical reprogramming system. By adopting a variety of strategies, more than 20,000 conditions were screened, and FCR was independently developed and created, which greatly accelerated the cell fate reshaping process, shortening the original process of about 40 days to 7-12 days, achieving a qualitative leap of 1 week at the fastest.
On this basis, we systematically demonstrated the transcriptional and epigenetic dynamics during FCR by using multi-omics sequencing and integration analysis, and revealed that FCR underwent a unique diapause similar state in the later stage. Interestingly, the authors also found a unique mechanism by which heterochromatin histone modification of H3K9me3 acts as a reprogramming barrier by regulating pluripotent endogenous retrovirus ERVs. In the process of FCR, cells feel foreign signals, overcome numerous obstacles, "break the cocoon into a butterfly", and turn around magnificently.

In order to solve the key problems such as long cycle and low efficiency of chemical reprogramming, doctoral student Chen Xi developed a unique approach and adopted a screening strategy different from previous studies. Taking the final generation of iPSC efficiency as the screening index, by screening about 7000 small molecules in the three stages of chemical reprogramming, it was found that a series of effective small molecules could significantly improve the reprogramming efficiency: in the first stage, SR11237, CD3254 and CCT129202 were found. TSA, TMP269, Citarinostat, DMH1, AZD9291 mesylate and SGC-CBP30 were found in the second stage, and small molecules Vc and WS6 were found in the third stage. Further tests showed that the window period of growth factor mLIF, small molecule VPA and R406 was 2 days, suggesting that the chemical reprogramming process could be subdivided into a stage every 2 days, with room for further optimization.
Subsequently, FCR was established by integrating small molecule library screening, concentration testing, treatment duration testing, single small molecule removal testing, etc., by adding small molecules with promoting effects into the chemical reprogramming system and adjusting their concentration and treatment time. The removal of any small molecule or growth factor at any stage of the FCR process results in a decrease in iPSC induction efficiency, indicating the necessity of these small molecules and growth factors for efficient reprogramming. Among them, the key pluripotent gene Oct4 has been activated on the 7th day of reprogramming, further demonstrating the rapidity of FCR. Through rigorous cellular, molecular and functional experiments, the authors demonstrate that the IPscs produced by FCR are fully pluripotent. These experiments prove that FCR is a fast, efficient and safe system to obtain iPSC by pluripotent reprogramming without dependence on exogenous transcription factors.
So, what kind of earth-shaking changes do cells undergo during FCR? To this end, the authors constructed a multi-omics library sampling 8 time points throughout the FCR process, including: RNA-seq, ATAC-seq, CUT&Tag (H3K27ac, H3K4me3, H3K18la, H3K27me3, and H3K9me3) and RRBS comprehensively explored the transcriptome and epigenome dynamics during FCR. The authors found that FCR and transcription factor reprogramming differ significantly in the reprogramming pathway, and further found that ectodermal related transcription factors promote the pathway difference between the two reprogramming systems. Transcriptome analysis showed that ribosome, splice, and DNA replication-related genes were significantly down-regulated in the late FCR period, suggesting that the late FCR period may experience a diapause similar state. In mice, diapause is a special blastocyst state induced by environmental factors, which leads to the slowing or even stagnation of blastocyst cell proliferation, the reduction of protein synthesis rate, and the delay of embryo implantation. This special mechanism contributes to the survival of offspring [14].
Mouse embryonic stem cells can also induce a diapause similar state by means such as inhibition of the mTOR signaling pathway, Myc gene knockout, or starvation, in which mouse embryonic stem cells show significantly reduced protein translation and cell proliferation rates [15,16]. In addition, some cancer cells have also been found to gain chemotherapy resistance by entering a similar state of embryonic diapause cells [17]. However, whether similar diapause states also exist in the process of somatic cell reprogramming to iPSC remains unknown. In this study, we compared the transcriptome data with in vivo diapause blastocysts and demonstrated that FCR undergoes a diapause like state unique to reprogramming at a later stage.
In order to eliminate the interference of cell heterogeneity on RNA-seq results, we performed RNA-SEQ analysis at day 0, 4, 8, and 12 of FCR and iPSC sampling, and found that FCR underwent fibroblasts, epithelioid cells, intermediate cells, neonatal iPSC, and iPSC stages successively, thus achieving a gradual reshaping of cell fate. Through SCENIC analysis and knockdown experiments, the authors found and demonstrated that Stat3 and Sox7 are important intermediate transcription factors of FCR. By using pseudo-time series analysis, the authors again demonstrated that diapause related genes were significantly down-regulated in cells at the late stage of the successful FCR reprogramming pathway, and the key genes Larp1, Slc17a5 and Prkaa1, which promote cells to enter the diapause state, were knocked down, demonstrating that inhibition of similar diapause states would lead to lower FCR efficiency. These results suggest that diapause similar state is an important intermediate state in FCR process and plays a key role in the establishment of cellular pluripotency network.
Next, the authors continued to conduct a joint analysis by integrating ATAC-seq and CUT&Tag data of various histone modifications, and found that most of the dynamic chromatin accessibility sites in FCR were highly dynamic, accompanied by the dynamic changes of corresponding histone modifications. Even when cells undergo a diapause like state, intense epigenetic remodeling occurs within the nucleus. Further at the genome-wide level, the authors found that heterochromatin's signature modification, H3K9me3, was enriched during FCR in gene-sparse regions of the genome that contained a large number of ERVs.
In the process of FCR, the expression pattern of ERVs also experienced a gradual dynamic change from MEF to iPSC, indicating that ERVs may also participate in and influence the reprogramming process. H3K9me3 has been shown to regulate ERVs in pluripotent stem cells. Therefore, the authors screened and found 24 IPSC-related ERVs regulated by H3K9me3 [18]. The classical Oct4-Sox2-Tcf-Nanog motif of pluripotent transcription factor was found in IPSC-related ERVs, suggesting that these ERVs might participate in the construction of pluripotent networks. Through knockdown and small molecule inhibition experiments, the authors demonstrated that inhibition of H3K9me3 methyltransferase can promote FCR, while knockdown of IPSC-related ERVs leads to reduced FCR efficiency. These results suggest that H3K9me3 acts as a barrier to FCR by inhibiting IPSC-related ERVs.
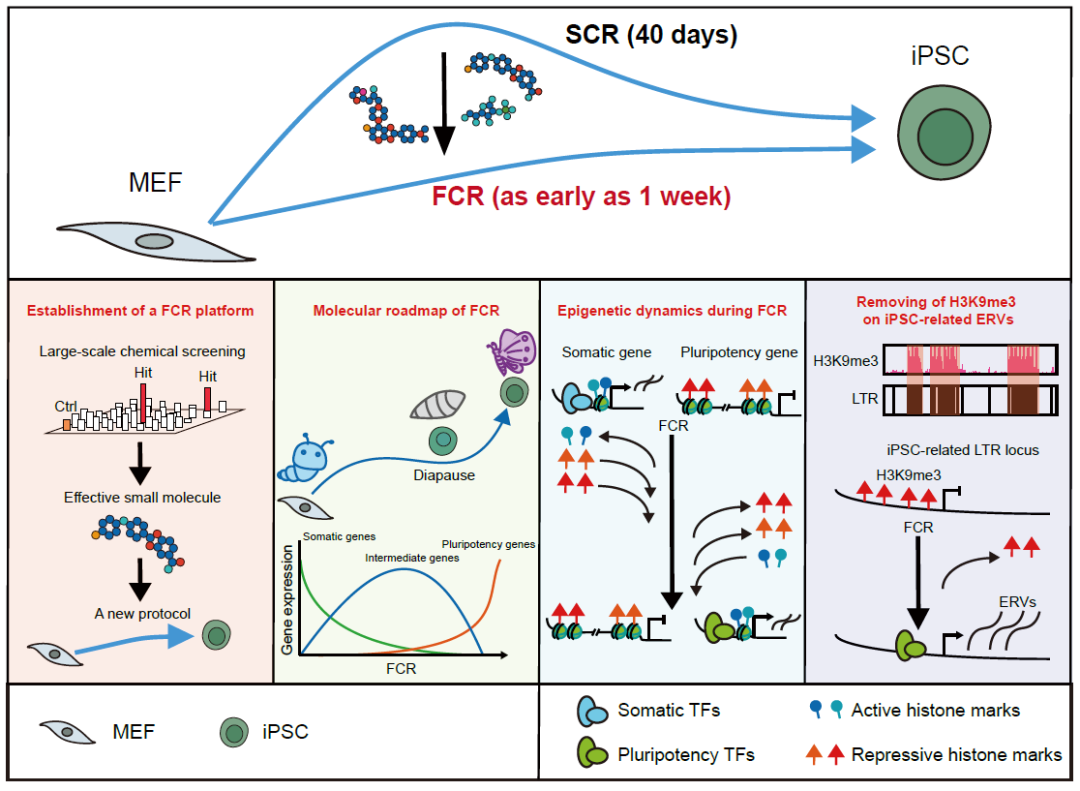 图. 研究模式图
图. 研究模式图
In general, this study adopted a new screening strategy to carry out large-scale small molecule screening, found a series of small molecules that can promote chemical reprogramming, and tested the optimal combination and concentration of these small molecules, and independently developed a new system for rapid chemical reprogramming of cells FCR. Multiomics integration analysis was used to systematically characterize the gene expression and epigenetic dynamics of the FCR process, finding that FCR undergoes a unique diapause like state later in life, and revealing that H3K9me3 obstructs FCR by inhibiting pluripotent ERVs (study model).
It is significant that this study provides an efficient and rapid cytochemical reprogramming technology platform, and reveals a unique cell fate remodeling mechanism, which will help researchers to further explore and understand the basic principles of cell identity establishment and maintenance. This study provides a theoretical basis and technical support for the clinical application of chemical reprogramming technology, which uses drugs to improve the plasticity of tissues with poor or weak regenerative ability, so as to be applied to regenerative medicine or to delay aging.
The research was supported by Prof. Li Wei, Prof. Holger A. Russ, Prof. Fu Junfen, Prof. He Xiangwei, Prof. Zhao Xiaoyang, Prof. Wang Hao and others.
Original link:https://www.nature.com/articles/s41556-023-01193-x