
Source: Cell Regen editorial Department
Human pluripotent stem cells (human pluripotent stem cells, hPSCs), including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), have the potential to differentiate into a variety of cells and tissues, and are one of the important sources of blood cells in vitro.
With the in-depth understanding of the mechanism of hematopoiesis, the efficiency of producing hematopoietic progenitor cells and progenitor cells from hPSCs also increases. However, it is still difficult to produce functional mature blood cells on a large scale by hPSCs and apply them in clinic.
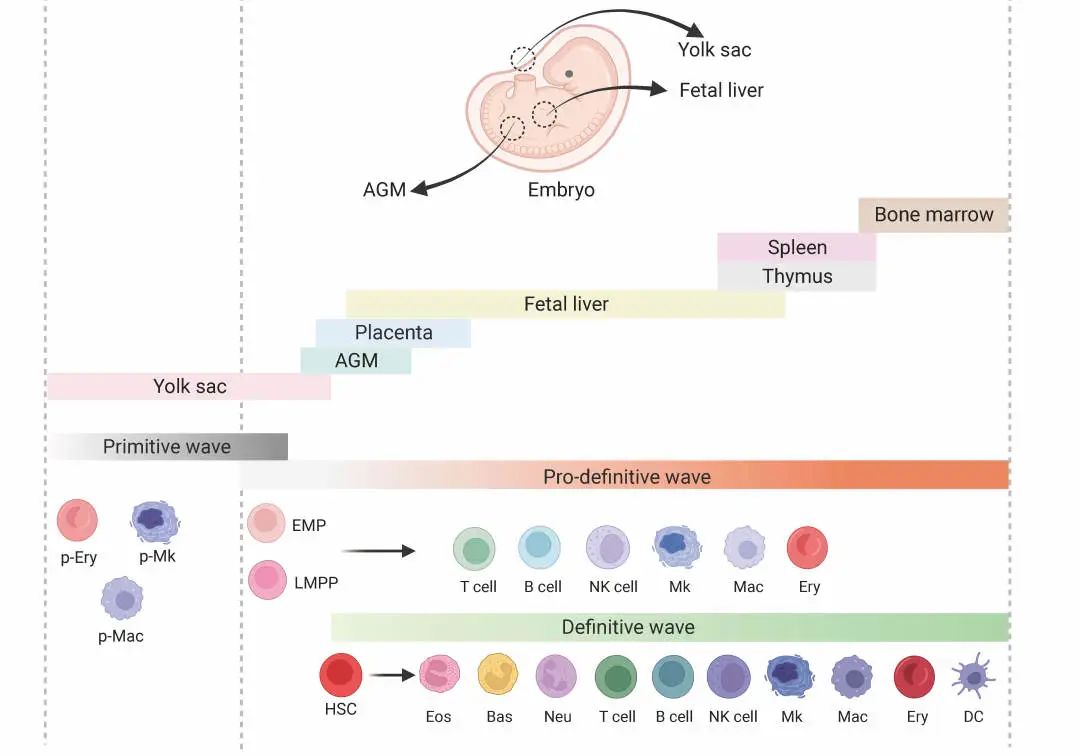
Schematic diagram of embryonic hematopoiesis in vivo
Recently, the Yellow River / Qian Pengxu Research Group of Zhejiang University published a review article entitled "Generating hematopoietic cells from human pluripotent stem cells: approaches, progress and challenges" on Cell Regeneration, which comprehensively summarized and compared the latest methods of producing hematopoietic stem / progenitor cells (hematopoietic stem/progenitor cells, HSPCs) and mature blood cells from hPSCs in recent years. In addition, the article also discusses the main challenges and possible solutions to the production of clinical grade blood cells.
Clinically, hematopoietic stem cells and terminally differentiated blood cells are always in short supply. HPSCs-derived HSPCs and mature blood cells have great potential in transplantation, immunotherapy, blood transfusion and other treatments. This paper summarizes in detail the methods of deriving HSPCs, lymphocytes, megakaryocytes / platelets, erythrocytes, macrophages and granulocytes from hPSCs.
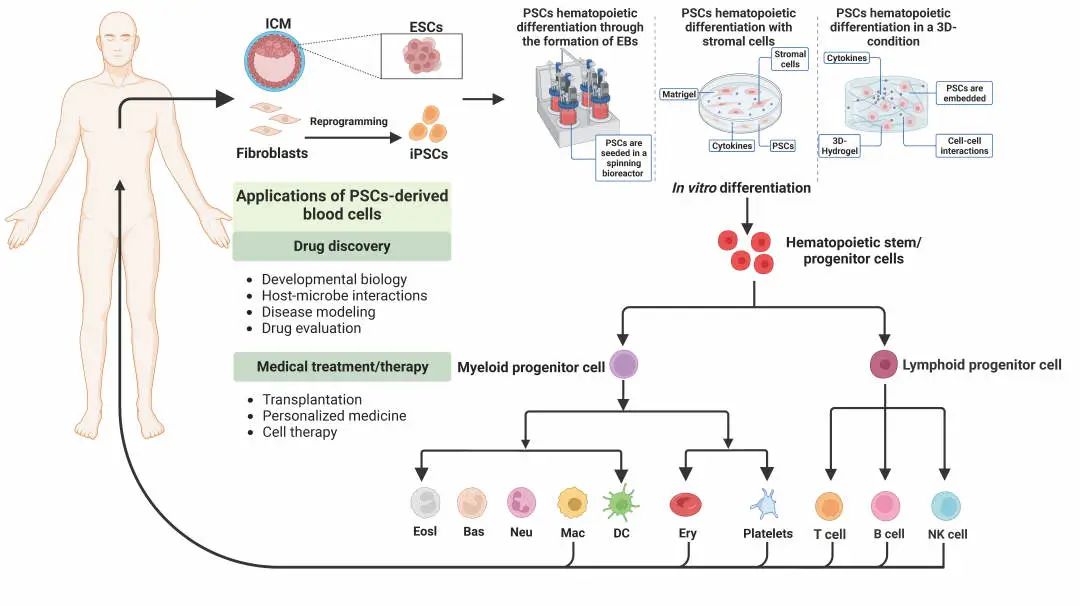
The method of inducing hPSCs to produce blood cells in vitro and the potential application of hPSCs-derived blood cells
At present, the methods of producing HSPCs in vitro include the formation of embryoid body, co-culture of stromal cell lines, addition of cytokines and small molecules, etc., but the culture environment of these methods is quite different from that in vivo. Recently, some studies have adopted a three-dimensional (3D) culture system, such as self-assembled peptide hydrogel can simulate the hematopoietic microenvironment in vivo to improve the efficiency of hematopoietic differentiation.Based on the research that hematopoietic stem cells originate from hematopoietic endothelial cells (hemogenic endothelium, HE), researchers induce hPSCs to HE, and then differentiate into functional HSPCs. This differentiation system well simulates the process of hematopoiesis in vivo and has a great application prospect. In addition, there are also studies on the formation of HSPCs through the formation of teratoma in mice.
At present, the in vitro differentiation system from hPSCs to lymphocytes is relatively mature, and the reinduction of hPSCs modified by CAR into corresponding CAR-T and CAR-NK also provides a new idea for cellular immunotherapy.
Compared with traditional CAR-T,CAR-NK, it has the advantages of no need for HLA matching, lower toxicity related to treatment and lower incidence of GVHD. Previous studies have shown that human ESCs and OP9 have the potential to differentiate into B cells in co-culture, but it is still challenging to produce functional B cells from hPSCs in vitro.
Platelet production can be increased by co-culture of HSPCs with OP9 or C3H10T1/2 stromal cells and regulation of key genes. In clinical application, in order to prevent blood transfusion mismatch, it is necessary to produce platelets with high universality. Recently, it has been studied that universal platelets are produced by knocking out HLA class I molecules from hPSCs by CRISPR/Cas9 technology. At present, the mature β-globin expression and enucleation rate of erythrocytes induced in vitro are very low. Therefore, exploring the difference between induced erythrocytes and naturally generated erythrocytes in vivo may be an important breakthrough.
Compared with lymphoid and erythroid lineages, myeloid differentiation in vitro has received less attention. Through the use of different combinations of cytokines, it is more suitable to produce specific types of myeloid cells. At present, hPSCs-induced macrophages have been used in cancer therapy-related research; hPSCs-induced mast cells can be used as a new clinical test system for allergens.
The unlimited proliferation ability of hPSCs has high clinical value, but it still has some limitations in application, such as tumorigenicity, immunogenicity and heterogeneity. In order to improve the safety of clinical application, we can improve the purity of hPSCs-derived blood cells or let hPSCs carry suicide genes.
At present, although there are many ways to increase the production and maturity of hPSCs-derived blood cells, mass production of mature blood cells suitable for clinical use is still difficult. To improve the maturity of hPSCs-derived blood cells, develop an economical, efficient and serum-free induction system with clear components, and use a bioreactor that can imitate the internal environment, it is expected to solve the transformation dilemma of hPSCs-derived blood cells and bring good news for patients with hematological diseases.