
Source: biological Valley
On the evening of September 4th, the research group of Gan Haiyun, Institute of synthetic Biology, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, published an article entitled "Destiny in the differentiation of Mouse embryonic Stem cells in symmetrical genetic Protection of parent histones" (Symmetric inheritance of parental histones contributes to safeguarding the fate of mouse embryonic stem cells during differentiation). The study found that the symmetrical distribution and inheritance of parental histones ensured the differentiation of mouse embryonic stem cells and early embryonic development of mice.

During the development of multicellular organisms, the genome ensures that genetic information is accurately transmitted to daughter cells through semi-reserved replication of DNA. Epigenetics is a heritable change in the state of gene expression when the genomic information is unchanged. Epigenetic information determines the fate of different cells and is the basis for establishing and maintaining cell type specificity. For eukaryotic cells, genome replication is closely related to the transmission of apparent information.
In the process of DNA replication, the nucleosome in front of the replication fork needs to be disassembled to ensure the smooth progress of the replicator.
After replicating the fork, the two newly synthesized sub-chains DNA need to recruit new synthetic histones and recycle parental histones from the mother chain to reassemble nucleosomes. The process of parental histone cycle and new histone supplementation is called DNA replication-coupled nucleosome assembly.
In recent years, researcher Gan Haiyun and his collaborators discovered the molecular chaperone that transmits parental histones in the process of DNA replication by developing eSPAN (enrichment and sequencing of protein-associated nascent DNA) technology, and revealed the molecular mechanism of parental histone inheritance.Mcm2 (a subunit of Mcm2-7 helicase) transfers parent histone to the lag chain of replicating DNA (1) through interaction with Ctf4 and Pola1. On the other hand, Pole3 and Pole4 (two subunits of lead chain DNA polymerase) promote the transfer of parental histones to lead chain (2) through interaction. During DNA replication, Mcm2-Ctf4-Pola1 and Pole3/Pole4 ensure the symmetrical distribution of parental histones and their post-translational modifications on sister chromatids (figure 1). However, how parental histones regulate apparent information transmission in DNA replication and its biological significance are not clear.
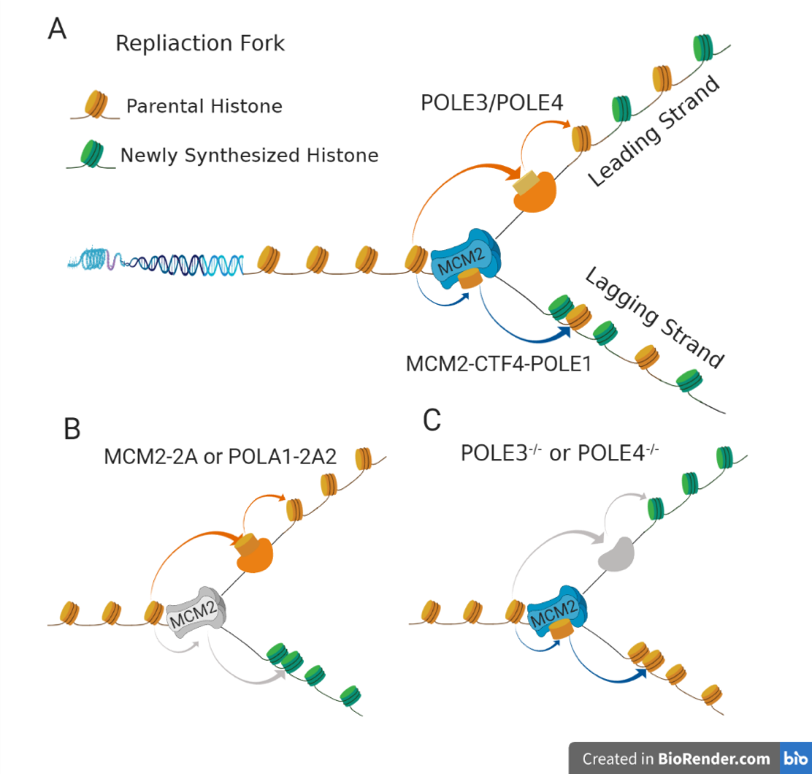
Apparent information transmission of parental histones by DNA replication coupling
Based on the previous discovery of MCM2 function, Gan Haiyun's research team obtained mouse embryonic stem cells without MCM2 histone binding function by point mutation (Mcm2-2A:Y81A/Y90A). Studies have found that the distribution of old histone (such as H3K27me3) is out of balance, the distribution of histone modification in the whole genome (such as H3K27me3, H3K9me3 and H3K4me3) is abnormal (figure 2), and the differentiation potential of embryonic stem cells into neural cells is reduced. Furthermore, through the construction of Mcm2-2A mice, it was found that the early embryonic development of homozygous mice was defective and the embryo died. The symmetrical distribution and inheritance of parental histones have protective effects on the differentiation of embryonic stem cells and early embryonic development of mice.
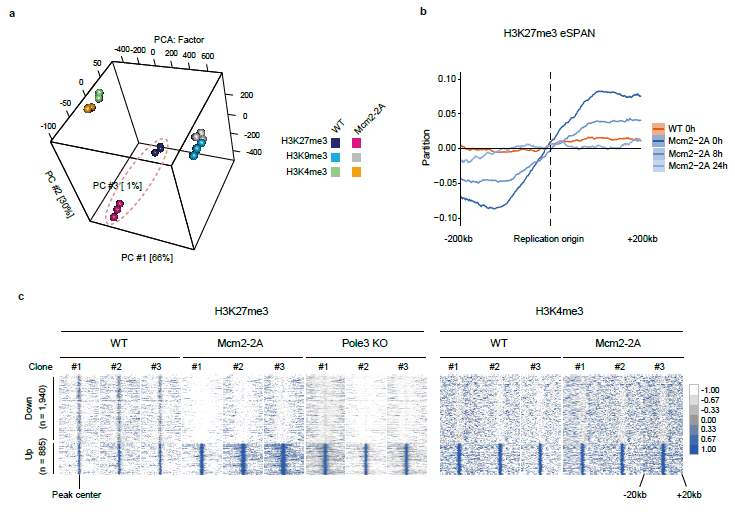
MCM2-2A mutation changes the landscape of H3K27me3 histone modification of embryonic stem cells
The modification carried by parental histones contains a lot of epigenetic information (such as H3K27me3, H3K9me3 and H3K4me3, which are closely related to cell differentiation and ontogeny). During the assembly of DNA replication-coupled nucleosomes, the newly generated chromatin contains about the same amount of parental histones and new histones, and half of the diluted histone modifications in daughter cells are restored to the original level by reconstruction, such as H3K27me3 reconstruction through the "read-write" mechanism.
The reconstruction speed of different histone modification is different. H3K4me3 can achieve rapid reconstruction, while H3K27me3 and H3K9me3 reconstruction is slow, which can not be completely recovered in one cell cycle. In the process of DNA replication, the imbalance of parental histone distribution may lead to different histone modification distribution in different daughter cells and possible changes in transcriptional level. Through single-cell H3K27me3 CUT&Tag, single-cell RNA-seq and LARRY-Barcode pedigree tracing techniques, it was found that Mcm2-2A embryonic stem cells had a tendency to differentiate into three germ layers in the system of neuronal differentiation.
The analysis showed that the heterogeneity of H3K27me3 distribution and transcriptome heterogeneity also increased (figure 3), which was inversely related to the transcriptional level of development-related genes and the H3K27me3 of this locus. The imbalance of parental histone distribution improves the gene expression and epigenetic heterogeneity of the daughter cell population, and affects the fate of the daughter cell.
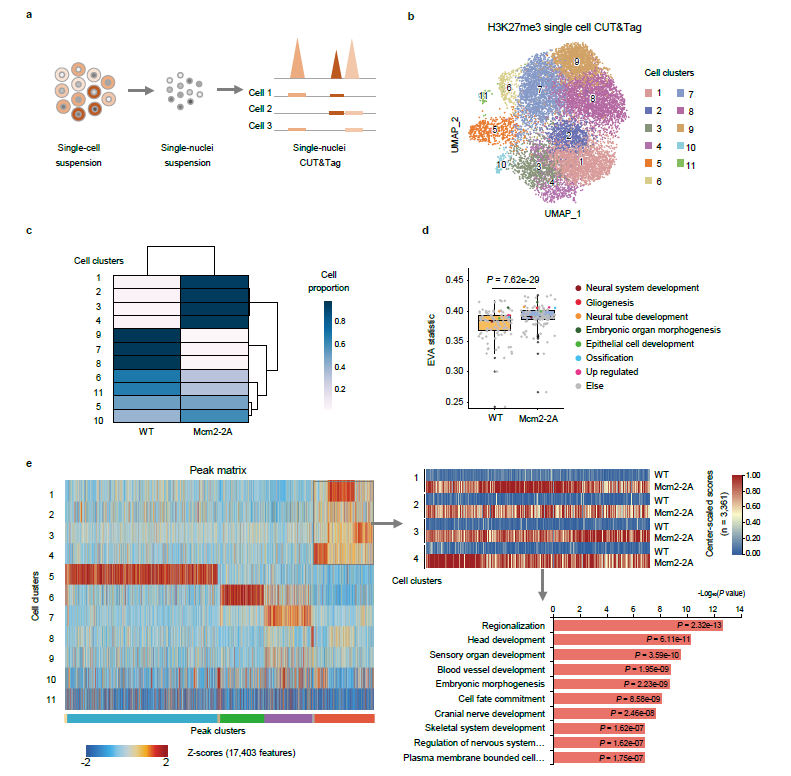
Mcm2-2A mutation enhances landscape heterogeneity of H3K27me3 histone modification in cell populations
At the promoter site of bivalent gene in mouse embryonic stem cells, the histone variant H3.3 participates in the establishment of correct H3K27me3 distribution pattern. It was found by eSPAN that H3.3 showed lag chain preference in Mcm2-2A embryonic stem cells. At the same time, the change of H3K27me3 distribution changed with the change of H3.3. The imbalance of parental histone distribution not only changes the distribution of slowly reconstructed histone modification, but also changes the location of histone variants, and then affects the landscape of histone and its modification in the whole genome, thus changing the fate of cells.
The results show that parental histone inheritance ensures the inheritance of histone modified landscape and contributes to the differentiation of embryonic stem cells and early embryonic development of mice. This finding has important scientific significance and potential contribution to understanding the distribution and inheritance of epigenetic information in offspring cells during cell division.
Original link: https://www.nature.com/articles/s41588-023-01477-w