
Source: BioArt
Inflammation and immune abnormalities are hallmarks of Alzheimer's disease (AD) and other neurodegenerative diseases. Microglia can trigger neuroinflammation, which leads to Alzheimer's disease, and adaptive immune cells also undergo phenotypic changes in Alzheimer's patients. However, adaptive immune cells exhibit both deleterious and protective effects in the pathogenesis of AD, which may be attributed to temporal regulation or functional heterogeneity of the adaptive immune system. On the other hand, intercellular communication is emerging as a key determinant of outcomes in diseases such as inflammation, infection and cancer, yet the regulation of this communication in the brain parenchyma and its contribution to neuroinflammation is poorly understood.
Recently, The Research team of Hongbo Chi and Wei Su (first author) from St Jude Children's Research Hospital in the United States published the CXCR6 orchestrates brain CD8+ in Nature Immunology T cell residency and limits mouse Alzheimer's disease pathology, brain resident CD8+ T cells co-expressing CXCR6 and PD-1, It is found in patch-associated microglia in the brains of people and mice with AD. The study also found that CD8+ T cells impeded AD progression, including amyloid beta deposition and cognitive decline. Ligand-receptor interaction analysis identified CXCL16-CXCR6 intercellular communication between microglia and CD8+ T cells. Cxcr6 deficiency impairs brain PD-1+CD8+ T cell accumulation, tissue retention, and clonal expansion. Loss of Cxcr6 or CD8+ T cells ultimately increases the production of pro-inflammatory cytokines in microglia, and CXCR6 regulates the colocalization of brain CD8+ T cells - microglia. Overall, the study reveals the protective role of brain CD8+ T cells and CXCR6 in the pathogenesis of AD in mice, and reveals microenvironmentally specific intercellular communication to coordinate tissue homeostasis and protect against neuroinflammatory damage。
Immune cell changes occur in the peripheral and central nervous system (CNS) of AD patients and in mouse models, but little is known about the changes in immune cells such as non-microglia in the brain parenchyma during AD. The researchers began by examining CD45int/+CD11b+ (mainly microglia) and CD11B + (mainly microglia) from the brain parenchyma (hereinafter referred to as brain) or meningeal dura and arachnoid tissue (hereinafter referred to as meninges) of 5xFAD and non-transgenic (Non-Tg) control mice CD45+CD11b- (non-microglial immune cells) cells were subjected to RNA-SEQ analysis and found that, as expected, disease-associated microglia (DAM) were limited to 5xFAD mice. Surprisingly, the frequency of CD8+ T cells increased in 5xFAD mice, but not in CD4+ T cells or γδ T cells. Cytomeflow and immunohistochemical results also showed that CD8+ T cell accumulation is very conserved in mouse disease models and AD patients, and this accumulation phenomenon is mainly specific to the brain parenchyma, but not in the meninges or other tissues in the lung and liver. By studying TCRα-deficient 5xFAD mice lacking CD8+ and CD4+ T cells (5xFAD; Selective loss of Tcra-/- mice) and CD8+ T cells (5xFAD; B2m-/- mice), found increased Aβ plaque burden and decreased memory function, confirming the protective effect of CD8+ T cells on the development of AD.
Intercellular communication networks affect tissue homeostasis in the central nervous system. However, little is known about the specific signals of intercellular communication in AD, especially for non-microglial immune cells. Analysis of a single chemokine ligand-receptor pair showed that CXCL16-CXCR6 was the most highly ranked ligand pair between 5xFAD mouse microglia and CD8+ T cells. In humans, the expression of CXCL16 is also altered in relation to AD, suggesting that the regulation of CXCL16 is consistent between mouse AD models and humans. The loss of CXCR6 resulted in a significant decrease of CD8+ T cells in the brain parenchyma of 5xFAD mice. CD8+ T cells in the meninges of Cxcr6-/- mice were reduced, but CD8+ T cells in the spleen, liver, and lungs were largely unchanged, so the effect was specific to the central nervous system. At the same time, CXCR6 deficiency was associated with a decrease in brain CD8+ T cells and an intensification of disease pathology in 5xFAD mice.
To further investigate how tissue specificity affects the accumulation and function of CD8+T cells, we performed paired analyses of scTCR-seq and scRNA-seq to identify clonal and amplified T cell populations in the brain and brain of 5xFAD and Non-Tg mice. The results showed that the clonal expansion of CD8+ T cells showed tissue and disease specificity. To identify molecules that can tag clone-expanded brain CD8+ T cells, the researchers calculated the Pearson correlation coefficient between the expression of a single gene and the frequency of clonotype, and found that Pdcd1 (coding for PD-1) was the gene most associated with clone-expanded cells. In addition, imaging analysis showed that CD8 and PD-1 were co-expressed in the brains of 5xFAD mice and AD patients. CD8+ T cell clonal amplification is more likely to occur in the brain than in the meninges, thus supporting CD8+ T cell clonal amplification with TRM cell phenotype with central nervous system zoning specificity and disease relevance. Together, these results suggest that CXCR6 is essential for TRM cell programming of brain CD8+T cells in the context of mouse AD, and reveal a key role of CXCR6 in linking brain CD8+T cell clone expansion and tissue residency programs.
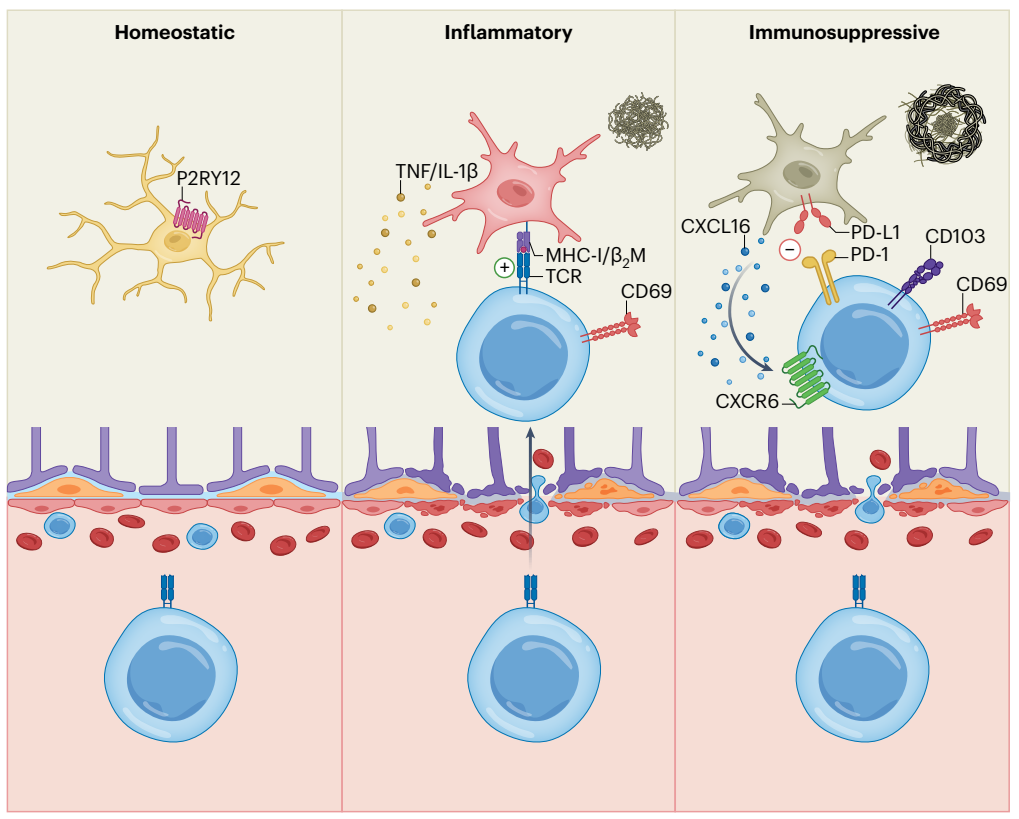
To investigate how CD8+ T cells are protective in the development of AD, high-resolution confocal imaging results showed that CD8+ T cells or CD3+ T cells were confocal with Iba1+ microglia near Aβ plaques in 5xFAD mice. CD8+ T cells were also observed in such close proximity to Aβ and IBA1+ microglia in the brains of AD patients. Thus, co-localization of CD8+ T cells with microglia or Aβ is A conserved phenomenon in mouse models and humans. At the same time, the loss or reduction of brain CD8+ T cells leads to an elevated inflammatory state of microglia.
It was found that Pdcd1+CD8+ T cells enriched with this regulatory CD8+ T cell feature, which was associated with increased expression of CD73, LAG3, CD39 and TIGIT, as well as CXCR6. In order to directly detect whether CD8+ T cells have inhibitory effects on microglia, PD-1+CD8+ T cells were generated from spleen CD44hiCD8+ T cells in an in vitro system and co-cultured with primary microglia. In the presence of PD-1+CD44hiCD8+ T cells, expression of TNF-α and pro-IL-1β was reduced in stimulated microglia, which was consistent with increased expression of immunosuppressive markers (as well as CXCR6) on cultured PD-1+CD44hiCD8+ T cells. Similarly, CXCR6+CD8+ T cells also showed this inhibitory effect. Brain-derived CD8+ T cells from 5xFAD mice also inhibited microglia from producing pro-inflammatory cytokines. However, the 5xFAD (5xFAD; Brain CD8+ T cells of Nt5e-/-) mice have certain defects in inhibiting the production of microglial proinflammatory cytokines in vitro, suggesting that CD73 may partially promote the inhibitory mechanism of brain CD8+ T cells. Therefore, PD-1+CD8+ T cells inhibit the production of proinflammatory cytokines in microglia.
In conclusion, this study highlights that the accumulation of tissue-residing CD8+ T cells plays A role in maintaining microglia functional balance in the presence of increased Aβ plaque load. This study suggests that Aβ plaque load may induce pro-inflammatory microglia proliferation, while CD8+ T cells accumulate at A later stage and play A balancing role in reducing the inflammatory state of microglia to limit further Aβ plaque formation and cognitive decline. The bidirectional interaction between microglia and CD8+ T cells mediated by CXCL16-CXCR6 and the immunomodulatory role of brain-resident CD8+ T cells may be important immunotherapeutic targets for neurodegenerative diseases.
It is worth mentioning that, at the same time, Kristen E. Funk of the University of North Carolina at Charlotte published a News & Views article CD8+ T cells pump the brakes on Alzheimer's disease, the above work was recommended.

Original link:https://www.nature.com/articles/s41590-023-01604-z