
Source: Cell plus
Traditionally, multiple sclerosis (MS) has had few treatment options.
Recently, By an international team of collaborators (University of Cambridge, Milanbicocca, Casa Sollievo della Sofferenza and S. Maria Terni Hospitals (Italy) and Ente Ospedaliero The study by Cantonale Hospital (Lugano, Switzerland), as well as the University of Colorado (USA), has shown that the injection of aborted fetal neural stem cells into the brains of patients with progressive multiple sclerosis (MS) is safe, well-tolerated and has long-term effects that protect the brain from further damage. (ClinicalTrials.gov: NCT03282760, EudraCT2015-004855-37)
In multiple sclerosis patients, the immune system begins to mistakenly attack the myelin sheath. As the myelin sheath gets more and more damaged, it disrupts the electrical signals that travel through the nerves, resulting in decreased mobility, balance, sensation, and muscle strength.
A global initiative led by the National Multiple Sclerosis Society has identified three distinct but overlapping paths to cure: (1) halting the progression of MS, (2) restoring lost function by reversing damage and symptoms, and (3) ending MS through prevention. The initiative also highlights the need to target core drivers of disease development that traditional DMT (disease-modifying therapies) cannot counteract, such as chronic central nervous system (CNS) compartmentalizing inflammation, immune failure myelin regeneration, and ongoing neurodegeneration.
Previous work by the Cambridge group has shown in mice that skin cells reprogrammed into brain stem cells, transplanted into the central nervous system, can help reduce inflammation and may help repair damage caused by MS.
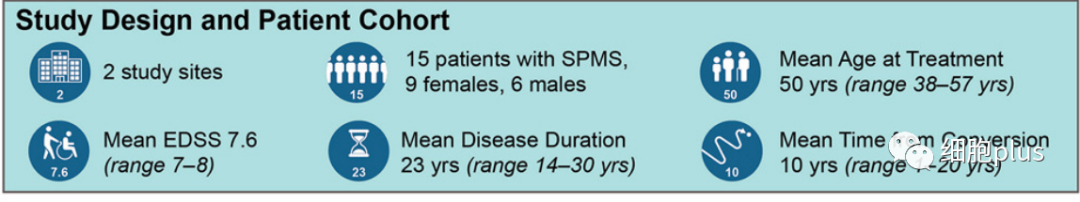
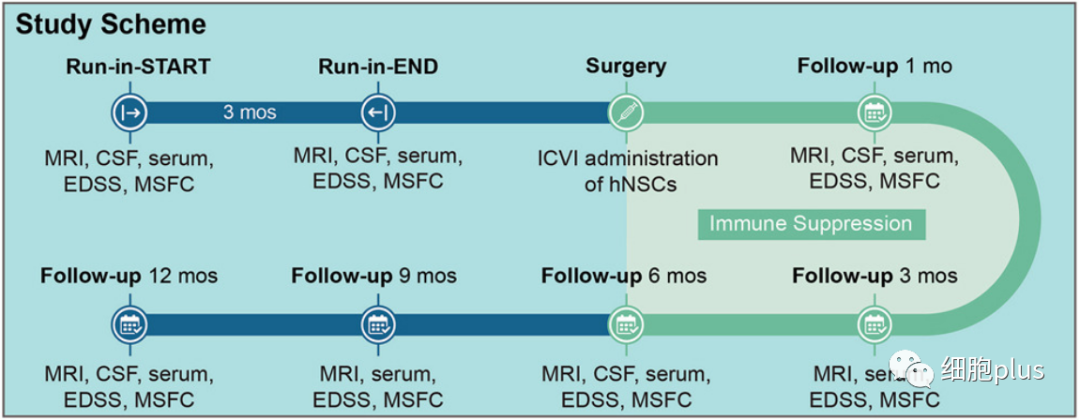
According to the clinical trial article published in CELL STEM CELL, the scientists completed this early-stage clinical trial, which involved injecting neural stem cells directly into the brains of 15 patients with secondary MS recruited from two hospitals in Italy (between September 26, 2017 and January 13, 2020). A total of 180 candidates volunteered for hNSC-SPMS.) .
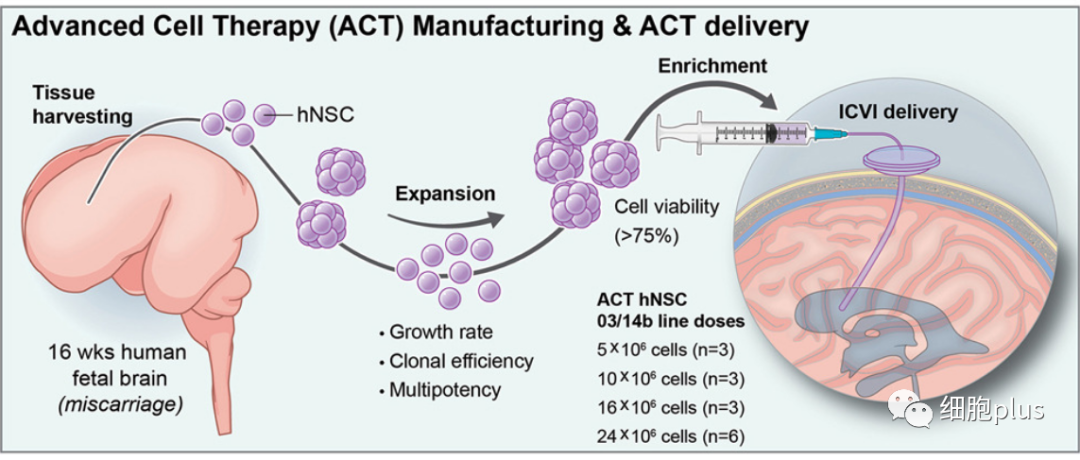
Stem cells are derived from cells taken from the brain tissue of a single aborted fetal donor. The Italian research team has previously shown that it is possible to generate an almost unlimited number of stem cells from a single donor, and that in the future it may be possible to obtain these cells directly from patients, which could help overcome practical problems associated with using allogeneic fetal tissue.
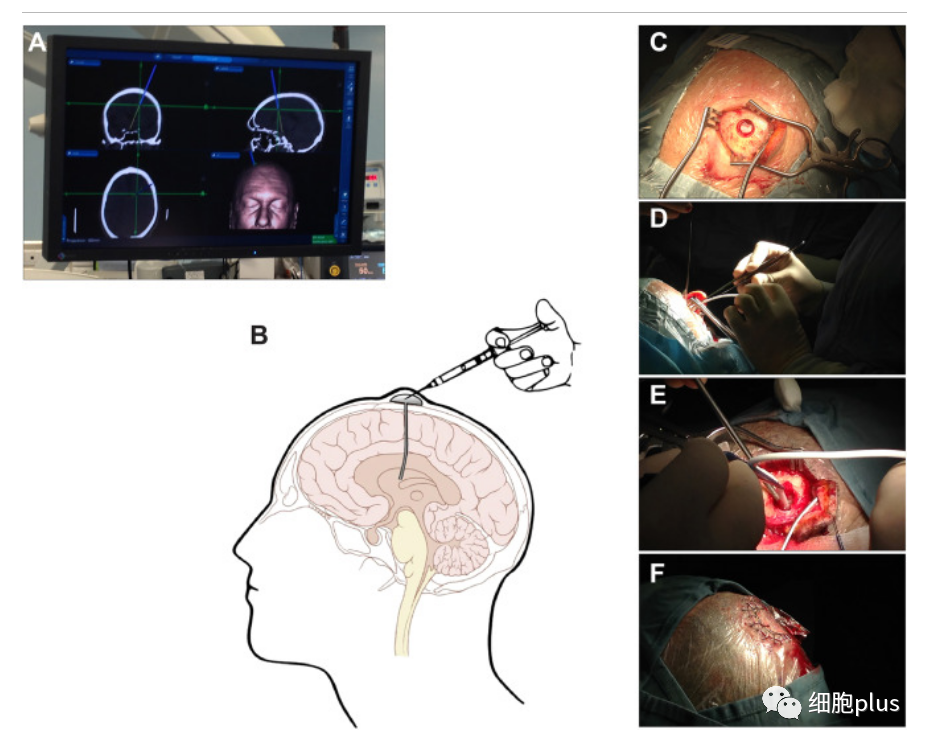
Prior to the surgery, the participants were given a comprehensive assessment of their disability and disease activity over a three-month period. Most patients treated at the time of transplant exhibit a high degree of disability (e.g., most require wheelchairs).
The researchers tested four doses of brain stem cells in combination with anti-rejection immune drugs. Encouragingly, participants experienced no serious adverse events in the 12 months following treatment, although there were some short-lived side effects (such as flu-like symptoms and respiratory infections).
Over the course of the study, none of the patients reported relapsing symptoms, nor did their cognitive function significantly deteriorate. Overall, the researchers say, this suggests that the disease is basically stable, with no signs of progression, because the high level of disability at the start of the trial made it difficult to expect such a good outcome.
The NMR results showed an association between higher stem cell doses and reduced brain volume, suggesting that the cells play a role in preventing inflammation and swelling in the brain.
The researchers assessed changes in the volume of brain tissue associated with disease progression in a group of patients. They found that the greater the dose of stem cells injected, the smaller the reduction in brain volume over time. They speculate that this may be because the stem cell transplant suppresses inflammation.
The team also looked for signs that the stem cells had a neuroprotective effect, that is, protecting nerve cells from further damage. Their previous work has shown that tweaking metabolism - how the body produces energy - can in turn reprogram microglia from "bad" to "good."
In the new study, they looked at changes in brain metabolism after treatment. They measured changes in the fluid and blood around the brain over time and found certain signs related to how the brain processes fatty acids. These symptoms are related to the effect of treatment and the development of the disease. The higher the stem cell dose, the higher the fatty acid levels, and this also continued for 12 months.
In particular, the trial aims to provide standardized HNSCS rather than unwanted, unidentified mixtures of brain cells, which is achieved through routine, detailed monitoring of a defined set of hNSC functional properties (EudraLex GMP for advanced therapeutic products: https://health.ec.europa).
A set of established HNSC-specific functional tests is routinely certified throughout each step of cell proliferation until transplantation to guarantee implantation of a pre-determined stable amount of hNSC. To complete the process, the more mature components of the culture were removed in the final selection step 48-96 hours before intervention, leaving only the highly immature clonogenic cells. The cells were transplanted within 20 minutes of harvest and had a survival index of 80% on average. Because the technology used in HNSC-SPMS is capable of continuous reproduction, not only the patients in this study, but also all participants in similar future trials will be transplanted with the same hNSC drug, which guarantees a stable and predictable composition and properties. This makes the availability of hNSCs in future clinical trials less of a limiting factor, so the authors believe that this approach is expected to significantly reduce the variability problem in future hNSC clinical transplantation.
The authors agree that the mechanism of action of this novel ACT drug is still undetermined, and it is not yet possible to document the survival, integration, and fate of donor cells after transplantation in human subjects.
Reference link:https://www.sciencedirect.com/science/article/pii/S1934590923003934#sec4