
Source: BioValley
As an in vitro research model with unique advantages, organoids have been used more and more widely in the biomedical field. Most organs have tissue-resident immune cells. Human organoids lack these immune cells, which limits their utility in simulating many normal and disease processes.
To address the above, researchers from Cincinnati Children's Hospital in the United States have co-developed multiple populations of immune cells by cultivating human colonic organoids (Hcos) derived from pluripotent stem cells, including hematopoietic endothelial (HE) -like cells and red bone marrow progenitor cells, which undergo typical differentiation steps to produce functional macrophages. The study is entitled "Development of functional resident macrophages in human pluripotent stem cell-derived colonic organoids and human beings. fetal colon, "in the journal Cell Stem Cell.
Different populations of immune cells exist in the gastrointestinal (GI) tract of adults, including bone marrow cells and lymphocytes. Most intestinal diseases, especially inflammatory bowel disease (IBD), are related to the immune system. Previous studies have suggested that most immune cells in the gut, including resident macrophages, are derived from bone marrow-derived hematopoietic stem cells (HSCS). However, animal studies have found that many organs contain a population of tissue-resident macrophages, which are produced independently of blood stem cells during embryonic development. Studies of embryonic development of tissue-resident macrophages in the colon of human fetuses have not been reported.
Despite separate advances in immune cell types and organoids derived from human pluripotent stem cells (hPSCs), the construction of human gastrointestinal organoids containing co-developing tissue-resident immune cells remains to be further investigated. In this study, the authors constructed human colonic organoids (Hcos) that differentiate into hematopoietic endothelium-like cells and a range of hematopoietic cell types.
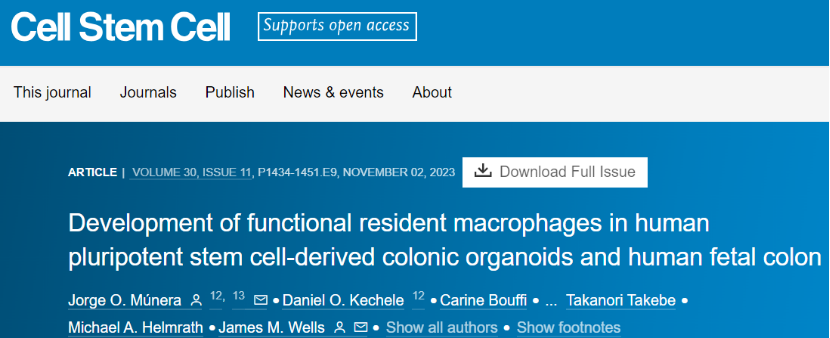
The researchers first constructed the human colonic organoid HCO. HCO contains colon epithelial and surrounding mesenchymal derivatives, including fibroblasts, myofibroblasts, and smooth muscle cells. RUNX1 marks hematopoietic progenitor cells in the hematopoietic endothelium, adjacent to the developing colon, and the aorta-gonado-mesonephric (AGM) region of the embryo itself is one of the major sites for the formation of HPC. The researchers found that in E10.5 mouse embryos, endothelial cells in the AGM region contained emerging RUNX1+HPC clusters. In addition, CD34+ cells were isolated from day 21 HCO and showed to undergo a process similar to endothelium-to-hematopoietic transition. Taken together, these data suggest that HCO cultures contain hematopoietic endothelium-like cells and associated RUNX1+ cells.
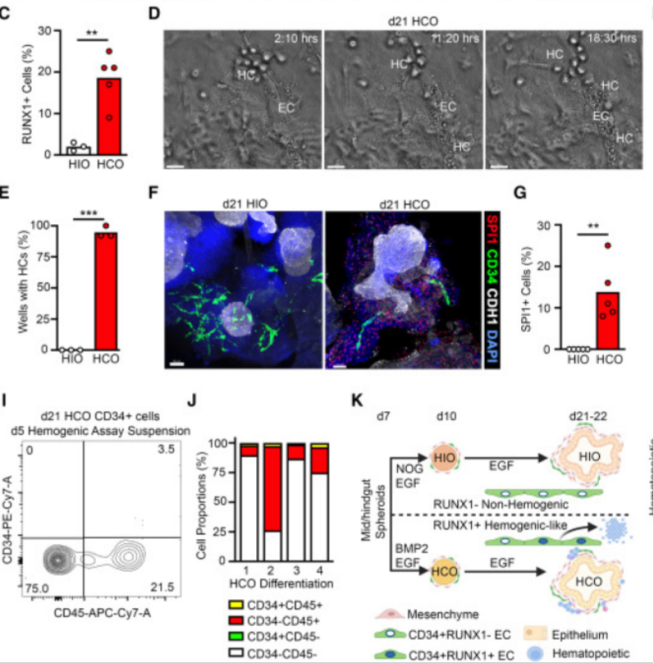
To characterize the hematopoietic potential in HCO cultures, the researchers performed transcriptional, morphological, and functional analyses. Giemsa staining was performed on cellular spinning cells harvested from HCO culture-medium at days 21-28 to identify primarily immune progenitor cells as well as monocytes and macrophage-like cells, as well as rare neutrophils, eosinophils, and basophilic cells. To examine red bone marrow progenitor cells (EMPs) in HCO, the researchers performed a Methocult assay and found that HCO cultures contained progenitor cells that formed ErP, bone marrow, and mixed bone marrow (MM) colonies. In summary, the early hematopoietic progenitor cells and lineages observed in HCO are very similar to those observed during the early hematopoietic process in human embryos.
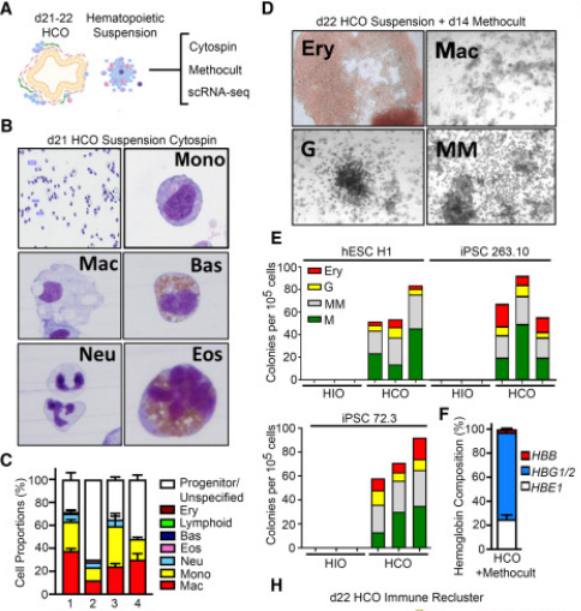
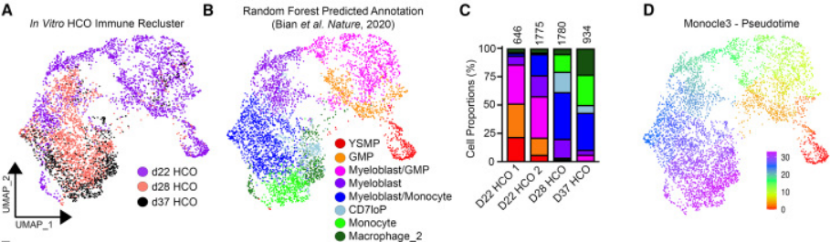
To determine whether HCO-associated hematopoietic cells differentiated into macrophages similar to those found in human embryos, the researchers performed RNA-SEQ analyses at three different time points during HCO macrophage development, i.e., day 22, 28, and 37. These three time points clustered in a continuum with a marked progression from progenitor cells to macrophages, and the differentiation of hematopoietic progenitor cells to macrophages in HCO was found to be transcriptionally similar to human fetal hematopoietic cells and to show a similar bone marrow differentiation bias.
Further studies found that macrophages that co-developed in HCO cultures in vitro acquired tissue-resident transcriptional signatures similar to their in vivo counterparts. The researchers found that a subset of HCO macrophages expressed inflammatory cytokines, and the addition of the inflammatory suppressor cytokine IL-10 inhibited the secretion of these pro-inflammatory cytokines, suggesting that the IL-10-mediated immune tolerance mechanism in vivo is conserved in HCO cultures, and that HCO-associated macrophages can produce a rapid and strong inflammatory response to LPS. Leading to epithelial necrosis.
By studying the phagocytosis of HCO macrophages, the authors found that macrophages from HCO are functionally resident macrophages, capable of responding to bacterial particles and live bacteria. Finally, the authors investigated whether these macrophages continued to remain resident during long-term HCO development, retransplanting them for 4 months, which were histologically and transcribed similar to the human fetal small intestine and colon, and finally the researchers found that HCO could produce self-sustaining macrophages that were not derived from HCS.

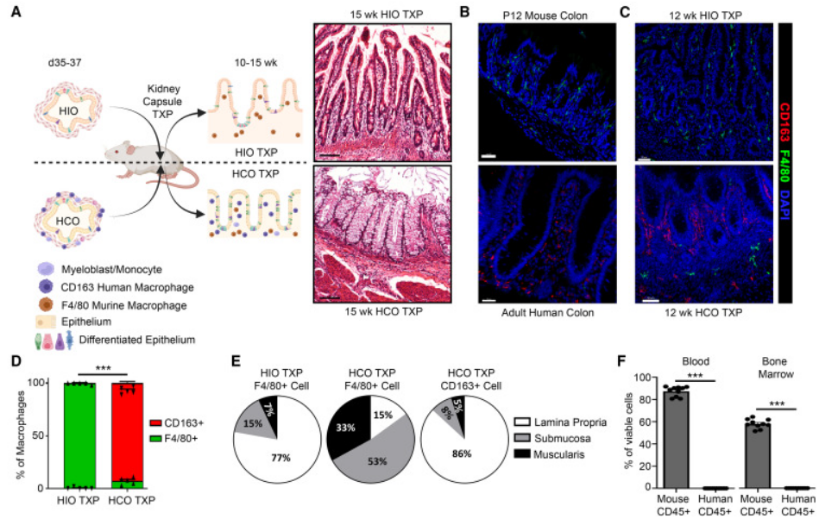
The data from this study suggest that the HCO environment provides the necessary clues for the development of pluripotent bone marrow progenitors, and transcriptional evidence supports the presence of pluripotent hematopoietic progenitors and lymphoprogenitors. The presence of these progenitors is short-lived because endogenous factors in the culture promote robust bone marrow differentiation from hematopoietic progenitors to monocyte progenitors to myeloblasts to ultimately monocytes and macrophages that persist in passing Hcos.
HCO macrophages acquired transcriptional characteristics similar to those of human fetal small and large intestine tissue-resident macrophages, which regulate cytokine secretion in response to pro-inflammatory and anti-inflammatory signals and are capable of phagocytic and strong responses to pathogens. When transplanted into mice, HCO macrophages remain within the colonic organoid tissue, establishing a close association with the colonic epithelium, and are not replaced by host bone marrow-derived macrophages. These studies show that HE in HCO produces pluripotent hematopoietic progenitor cells and functional tissue-resident macrophages. Together, this study provides a framework for generating other human organoid systems containing resident immune cells.
Reference:Múnera JO, Kechele DO, Bouffi C, et al. Development of functional resident macrophages in human pluripotent stem cell-derived colonic organoids and human fetal colon. Cell Stem Cell. 2023 Nov 2;30(11):1434-1451.e9. doi: 10.1016/j.stem.2023.10.002. PMID: 37922878.