
Source: BioArt
Scientists have been studying Hematopoietic stem cells (HSCs) for many years, but to date, they have not succeeded in developing carrier-free and transplantable HSCS. In contrast, many blood cells that can be used to treat disease, such as red blood cells, B cells, T cells, bone marrow cells, megakaryocytes, and hematopoietic cells, They can be produced from human embryonic stem cells (hESCs) or human induced pluripotent stem cells (hiPSCs). Recent studies have focused on obtaining populations of CD34+ CD45+ cells from pluripotent stem cells similar to adult HSCs used in clinical transplantation, but the limited transplantable ability of these cells in immunosuppressed mice has limited its use in therapy. To date, the only studies that have succeeded in generating true HSCs have been limited by the presence of plasmids or trophoblast cells encoding oncogenes and have not been able to achieve clinical application. Interestingly, HSCs with transplantable properties can be obtained from teratoma, suggesting that it is possible to generate HSCs from pluripotent stem cells. In fact, during embryonic development, endothelial cells in the aorta-gonad-mesonephros (AGM) region can also generate transplantable HSCs, These cells mimic the hematopoietic development in embryoid body (EB) through the formation of endothelium-hematopoietic transition (EHT).
On December 7, 2023, Thierry Jaffredo of the French Blood centre EFS and Laurence Guyonneau-Harmand of the Sorbonne University in Paris published a paper in the journal Cell Stem Cell entitled Generation of transgene-free hematopoietic stem cells from human induced pluripotent stem cells. Hematopoietic stem cells from human induced pluripotent stem cells. The researchers successfully developed a single-step culture system to obtain carrier-free and stromat-free HSCs from hiPSCs. After transplantation into the bone marrow of mice, these HSCs were able to settle and form multi-line hematopoietic cell populations, and could be transplanted continuously. The study revealed that the reconstructed HSCs were similar at the transcriptional level to HSCs derived from human embryos aorta. The results of this study are of great significance for further understanding the mechanism of HSCs formation.

HSCs are rare cells with lifelong therapeutic effect in Hematopoietic cell (HC) transplantation. Patient demand for HSCs has increased significantly in recent years, making HSCs transplantation a major challenge. The researchers hope to develop a carrier-free and stromat-free system from hiPSCs to generate human HSCs with strong, long-term multiple potential to recompose and self-renew. The idea is promising, but has yet to materialize.
Establishment of culture conditions
First, through an experimental design approach, the researchers identified ten combinations of cytokines and growth factors. They evaluated measures such as cell expansion, the frequency of long-term culture-initiating cells (LTC-ICs), and the expression of cell surface markers, identifying three effective combinations. Results of the study of three combinations for hiPSCs cell line differentiation found that combination A exhibited the best ratio of immature and mature hematopoietic markers. In NOD/SCID mice, combination A produced the highest proportion of human CD45+ cells. Therefore, the researchers chose combination A for follow-up study and application.
Characterization and functional characterization of hiPSCs differentiated cells
The researchers differentiated hiPSCs into CD34+CD45+ vascular endothelial cells and transplanted them into mice to assess their reconstructive hematopoietic potential. The results showed that the transplanted cells were able to successfully rebuild hematopoietic blood, similar to the effect of transplantation using CD34+ cells from cord blood. At 20 weeks after transplantation, a variety of human blood cells, such as myeloid, B cells and T cells, were produced in the bone marrow of the mice, showing good multiline differentiation. In addition, the study confirmed the presence of transplanted cells in peripheral blood circulation, showing a multiline pattern similar to that in peripheral blood of cord blood transplant recipients. In conclusion, the results suggest that blood cell remodeling can be effectively achieved through the differentiation of hiPSCs. Next, the researchers analyzed and functionally validated the cells transplanted into the bone marrow of mice. Various types of human cells, such as red blood cells, B cells and T cells, were found in the bone marrow of mice after transplantation. Functional verification showed that the transplanted cells exhibited normal blood cell properties, such as hemoglobin switching, T cell activation, and proliferation. Blood progenitor cell analysis confirmed the existence of functional human hematopoietic stem cells and their ability to sustain regeneration was confirmed by secondary transplantation experiments. Further experiments demonstrated that human hematopoietic stem cells obtained from EB cell progeny showed strong self-renewal potential and were able to steadily regenerate a variety of cells. These findings reveal the potential for blood cell reconstruction through cell transplantation.
EBs simulated HSCs development process analysis
Using transcriptome analysis and single-cell RNA sequencing (scRNA-seq), the researchers tracked the development of HSCs simulated by EBs. They observed the presence of 27 different cell types in the EBs at different time points in culture, distributed in three main branches corresponding to the ectoderm, mesoderm, and endoderm, as well as a fourth branch containing the trophoblast progenitor cell lineage. The researchers focused on endothelial and vascular HC cell populations and compared them to human embryo data on scRNA-seq. They found that the differentiation process produced a population of cells similar to embryonic aortic and venous endothelial cells, demonstrating that this differentiation process mimics the development of HSCS. By studying the expression patterns of MYB and RUNX1 genes, as well as flow cytometry analysis of endothelial markers CD34 and CDH5, the researchers further validated this process. The study also found that there is a dense network of blood vessels around EB, and there may be a transformation process from endothelial cells to HSC. Finally, the researchers identified the HSC-producing subpopulations by using six-gene markers and HSCs scores, and found that the phenotypes of the candidate HSC subpopulations were intermediate between early AGM and mature HSCS. Thus, molecular and phenotypic identification of EBs demonstrated that this in vitro culture process can reproduce the development of human HSCS, and provided detailed observation and analysis of endothelial and HSC cell populations.
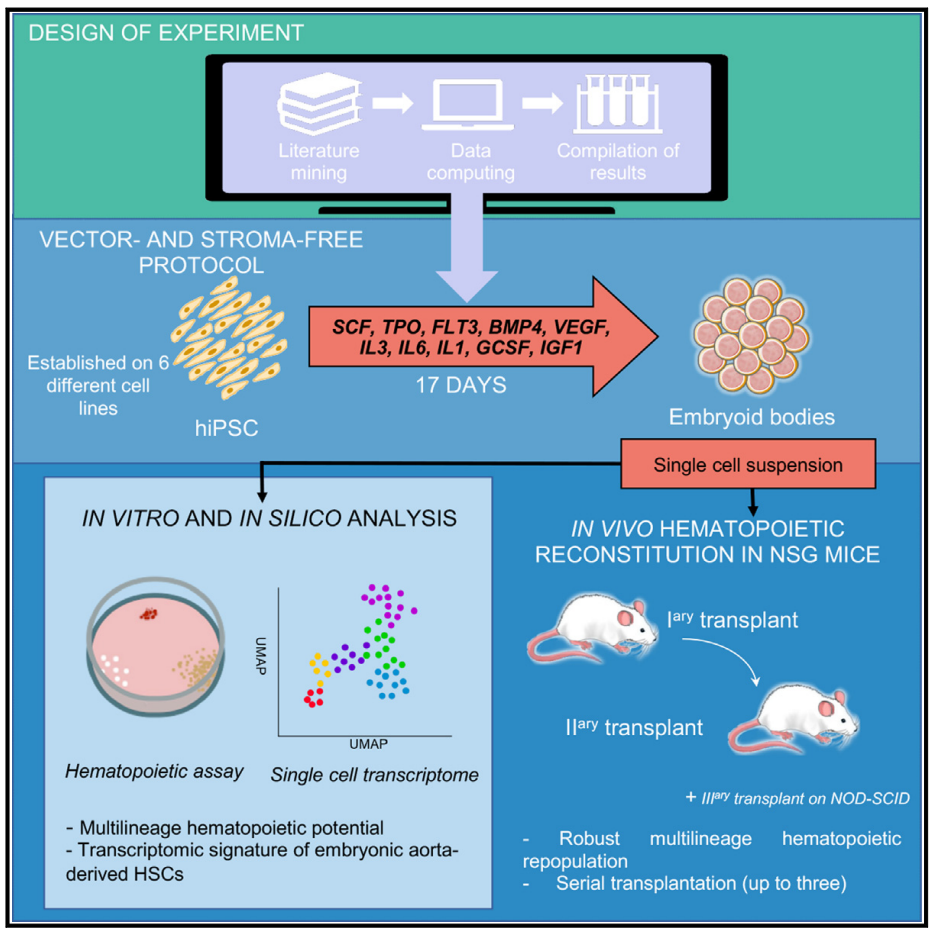
In summary, the study developed a carrier-free and stromat-free system to generate human HSCs with robust, long-term multipotential reconstitution and self-renewal capabilities from hiPSCs. The method uses EB formation to generate cell populations that are similar to immature HSCs found early on in the AGM region of human embryos. This suggests that the generation of carrier-free and stromat-free human HSCs is possible and can be achieved by producing cells that more closely resemble the original HSCs that emerge during development.
Original link:https://doi.org/10.1016/j.stem.2023.11.002