
Source:BioArtMED
Cell therapy is a good way to treat Parkinson's disease (PD) because its pathogenesis is relatively simple, namely local degeneration of dopaminergic neurons in the midbrain substantia nigra pars compacta region. In animal and human studies, the transplantation of ventral mesencephalon tissues or cells in human fetuses can restore motor function to a certain extent, but the parallelism of the results is poor, suggesting that this type of cell therapy needs to be improved. To ensure adequate number of midbrain dopaminergic (mDA) cells obtained, preparation and transplantation procedure standard.
Recent technological advances have enabled embryonic stem cells from human pluripotent stem cells (hPSCs), such as embryonic stem cells (embryonic stem cells), Potent generation of mDA neurons from ESCs and induced pluripotent stem cells (iPSCs) has been possible, which has laid a foundation for the establishment of HPSC-based transplantation therapy for PD. Multiple studies have confirmed that HPSC-derived mDA precursor cells integrate well into the host brain and mature into functional mDA neurons when transplanted into animal models of Parkinson's disease, significantly improving motor function. In addition, a recent pioneering study reported that PD patients who received autologous grafts of HiPSC-derived mDA precursor cells showed stable or improved clinical symptoms within 24 months of implantation. Currently, hiPSC sources (UMIN000033564, Japan) and hESC sources (NCT04802733, USA; NCT05635409 (EU) mDA precursor cells have recently entered early stage clinical trials.
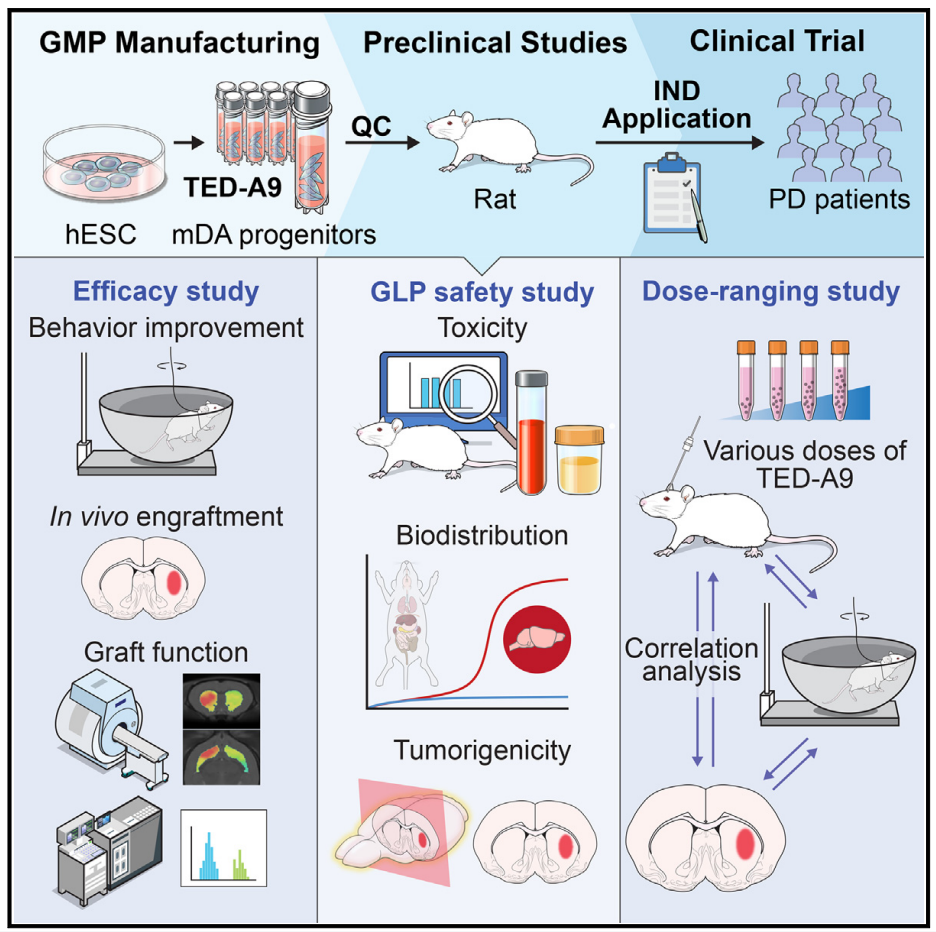
The first key step in the introduction of PD cell transplantation into the clinic is the precise and complete definition of cell product characteristics. It is particularly intuitively important to establish that cells are manufactured from clinical-grade materials, following GMP (good manufacturing practice) standards, and in accordance with clinical application standards. Subsequently, preclinical studies should provide strong evidence of efficacy through large-scale animal studies, and systematically and long-term focus on safety-related issues such as toxicity, biodistribution, and tumorigenicity. In addition, preclinical studies need to establish the optimal range of dosages for transplanted cells to serve as a reference for clinical trials. Most importantly, the study design must be careful, and the results must be evaluated in an unbiased manner. This usually involves working with a third party and being regulated throughout.
On December 11, 2023, Dong-Wook Kim, from the Yonsei University School of Medicine in South Korea, and Dae-Sung Kim, from Korea University, collaborated on the paper titled Preclinical and dose-ranging assessment of hESC-derived in Cell Stem Cell dopaminergic progenitors for a clinical trial on Parkinson's disease, presenting the process and results of a preclinical study aimed at introducing HESC-derived mDA neurons into human trials for the treatment of PD.

The cell differentiation methods in this study have been optimized for clinical application to ensure compliance with GMP standards. This optimization method makes it possible to mass-produce high-purity, cryopreserved mDA precursor cells while maintaining strict quality control (QC). A 1-year long-term large-scale transplant study using immunodeficient rats by an independent CRO showed, in addition, that clinical mDA precursor cells showed therapeutic potential and produced a dose range of therapeutic effects in toxin-induced semi-parkinsonian rats. These findings provide important information about appropriate cell dosages in human trials.
hESC derived mDA cell transplantation has great clinical prospect in the treatment of PD. The authors optimized the mDA preparation method under strict GMP conditions, and prepared clinical grade hESCs on a large scale, and obtained high purity and cryopreserved mDA precursor cells from them. The authors also evaluated the toxicity, biodistribution, and tumorigenicity of these cells in GLP (good laboratory practice) compliant facilities. At a third-party facility, mDA precursor cells in varying doses were transplanted into Parkinson's rats and observed for up to a year. The results showed that the transplanted mDA neurons did not have tumorigenicity, significant toxicity, or ectopic integration except at the injection site. Significant dose-dependent behavioral improvements were observed, with a minimum effective dose range of 5,000 to 10,000 mDA precursor cells. These results provide a strong basis for determining the minimum cell dose (3.15 million cells) for human clinical trials.
Based on the above results, the authors obtained the approval of PD cell therapy phase 1/2a clinical trial from the Ministry of Food and Drug Safety of Korea and have started clinical trials.
Original link:https://doi.org/10.1016/j.stem.2023.11.009