
Source: Boyah Life
In recent years, stem cell therapy has been gradually applied in the treatment of osteoarthritis (OA), which is expected to become a new generation of treatment for osteoarthritis. At the same time, the potential of stem cells to treat osteoarthritis continues to be demonstrated. A Phase I/II randomized controlled trial [1] published in Stem Cells Translational Medicine reconfirmed the great potential of stem cells in the treatment of osteoarthritis, showing that patients with repeated injections of mesenchymal stem cells showed more stable improvement in symptoms than patients with a single dose.
Osteoarthritis (OA) is currently occurring at an increasing rate and is characterized by joint degeneration and pain. Current traditional treatment options aim to relieve symptoms and slow disease progression, but lack cure potential. Previous pathophysiological studies have shown that the occurrence of osteoarthritis is closely related to joint degenerative changes and the inflammatory environment in the joint cavity. Therefore, promoting articular cartilage regeneration and improving the intraarticular inflammatory environment are important targets for the treatment of osteoarthritis.
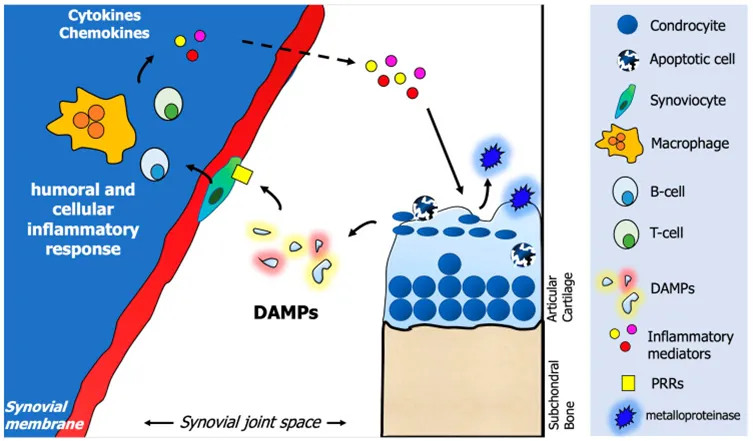
Involving multiple mechanisms, stem cells have the ability to delay cartilage damage and pain
Mesenchymal stem cells (MSCs) have cartilage differentiation and immunomodulatory properties, and therefore have potential value in the treatment of osteoarthritis.
The strong immunomodulatory properties of MSCs and the secretion of a variety of cytokines can regulate the inflammatory environment of joints in patients with knee osteoarthritis.
Mesenchymal stem cells also have a unique ability to induce the growth of new chondro-like cells, making them suitable for use in knee cartilage repair.
At the same time, the exosomes of MSCs can also play a repair role through paracrine and intercellular interactions [3].
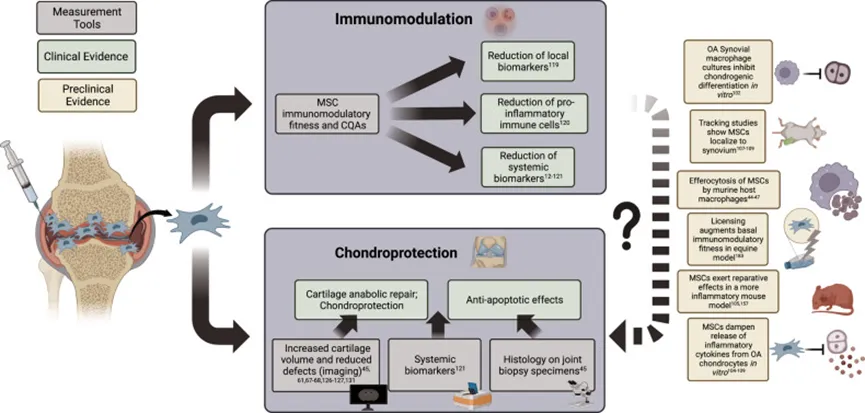
Use Case: Patients with multiple transfusions of mesenchymal stem cells showed significant improvement in pain and function
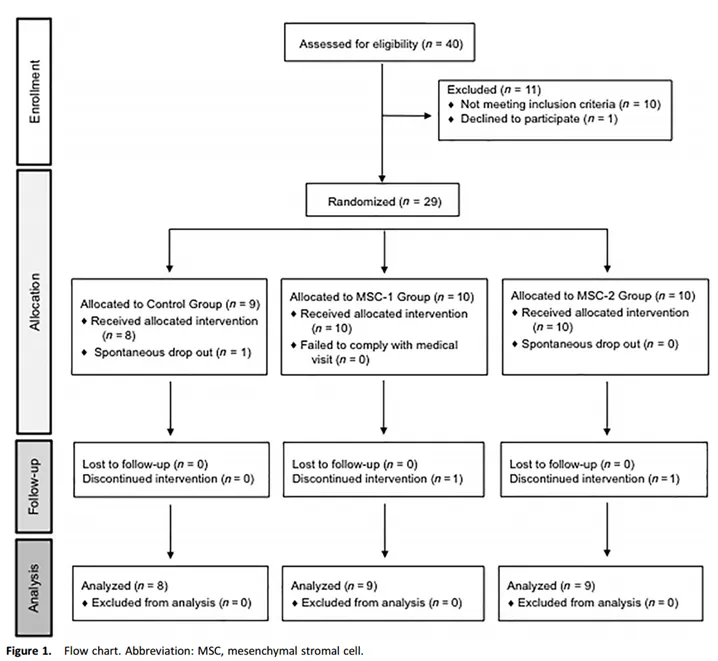
The Phase I/II randomized controlled trial, published in Stem Cells Translational Medicine, evaluated the safety and efficacy of a single or repeated intracavitary injection of umbilical cord mesenchymal stem cells (UC-MSCs) in the treatment of knee osteoarthritis (OA).

The study ultimately enrolled 29 patients who were randomly assigned to one of three study groups:
1. Control group (n= 8) : received intra-knee injection of hyaluronic acid (HA) at baseline and at 6 months.
2. MSC-1 group (n = 9) : Only UC-MSCs were injected into the knee at baseline (dose: 20 × 10 ^ 6) with placebo at 6 months.
3. MSC-2 group (n = 9) : Received intra-knee injection of UC-MSCs at baseline and at 6 months (dose: 20 × 10 ^ 6).
The safety and efficacy of UC-MSCs in the treatment of knee osteoarthritis (OA) were comprehensively evaluated by the clinical symptoms and imaging (MRI) changes of patients during a 1-year follow-up.
01.Umbilical cord mesenchymal stem cells are safe
There were no serious adverse events during the trial. The most common adverse event associated with intra-articular injection is acute synovitis. One week after the first injection, mild-to-moderate symptomatic knee effusion was more common in the cell therapy group than in the control group, but there was no significant difference. Pain was the second most common adverse event, but there was no statistical difference between groups. Both are transient and can be cured with rest or oral acetaminophen without hospitalization or joint aspiration. These results indicate that single/repeated intra-knee injection of UC-MSCs is safe and feasible.
02. Multiple injections of umbilical cord mesenchymal stem cells obtained good clinical outcomes
The researchers tracked the evolution of symptoms in each group in a timely manner throughout the study and found that patients treated only with MSC showed significant improvements in pain and function from baseline compared to patients in the control group.
At 12 months, patients with repeated mesenchymal stem cell administration (MSC-2 group) experienced an 86 percent reduction in pain and an 89 percent reduction in disability, compared to 38 percent and 50 percent in the control group, respectively. A single mesenchymal stem cell administration (MSC-1 group) continued to improve during the first 9 months, reaching similar levels of symptoms as the control group at 12 months. In contrast, patients with repeated mesenchymal stem cell transfusions had stable improvements in both WOMAC and VAS up to the end of the study.
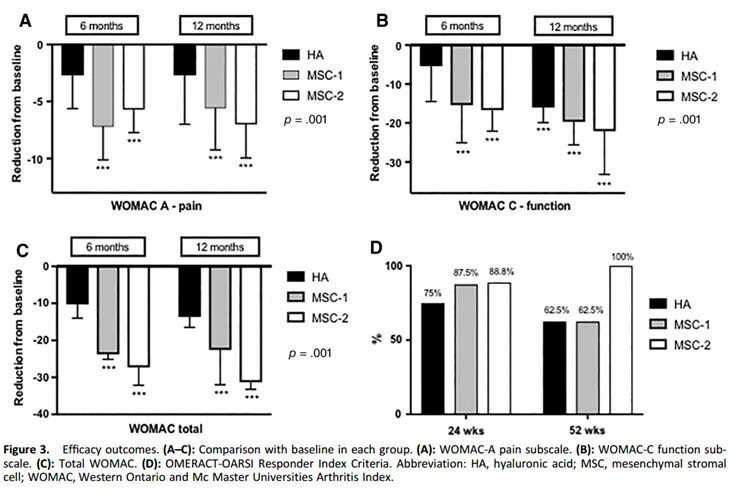
Specifically, both the MSC-1 and MSC-2 groups had significant pain and functional improvements compared to the control group. The pain subscale of the WOMAC Scale, which is used to assess the severity and treatment response of patients with arthritis, showed that the level of pain in the MSC-2 group was significantly lower than that in the control group at 1 year of follow-up.
In addition, the Visual Analog Scale (VAS), a subjective scale used to assess the degree of pain in patients, showed that the VAS scale score in the MSC-2 group was significantly lower than that in the control group at 1 year follow-up (2.4 ± 2.1 vs. 22.1 ± 9.8, p = 0.03). This means that repeated administration of mesenchymal stem cells can significantly improve patients' pain. Taken together, the replicated umbilical cord mesenchymal stem cell administration strategy in this phase I/II trial has a good safety profile and clinical outcomes for the treatment of long-term pain in patients with osteoarthritis.
Brief summary
Osteoarthritis has been called the "cancer of death", the increasing incidence and limited treatment options are the current challenges. The emergence of stem cell therapy provides a solution to the above dilemma. Intra-articular injection of mesenchymal stem cells can significantly reduce the pain of knee osteoarthritis patients, restore the motor function of patients to a certain extent, and its safety has been proven. Various clinical studies have shown great potential for MSCs in the treatment of osteoarthritis, but more randomized controlled clinical trials and long-term follow-up of patients are needed to further advance the clinical use of stem cell therapy for osteoarthritis.