
Source: Biological Exploration
Blood exosomes are emerging as potential biomarkers for the diagnosis of brain diseases such as Alzheimer's disease (AD). There is currently a lack of an ultra-sensitive technique to identify core AD biomarkers in blood exosomes to optimize the use of biomarkers in clinical practice.
On March 27, 2024, Luo Haiming's team at Huazhong University of Science and Technology published a Science Advances paper entitled "Sensitive detection of multiple blood biomarkers via immunomagnetic exosomal PCR. "the diagnosis of Alzheimer's disease," which developed an immunomagnetic exosome polymerase chain reaction (iMEP) platform, Rapid detection of amyloid-beta (Aβ1-40 and Aβ1-42) and phosphorylated tau (p-tau396,404, and p-tau181) in clinical blood exosomes using DNA-coupled antibodies.
Toehold shift mediated DNA affinity drawdown eliminates the high detection background, allowing biomarkers to be detected at concentrations as low as 10 Fg/ml. Using the iMEP assay, exosome Aβ1-42 was more accurate in distinguishing between AD patients and healthy individuals, with A sensitivity of 95.0% and specificity of 95.0%, compared to exosomes p-tau181 and P-TAU396.404. iMEP technology is also adept at quantifying levels of different exosome biomarkers associated with disease pathogenesis.
Alzheimer's disease (AD) is A progressive neurological disorder characterized by amyloid protein (Aβ) plaques and neurofibrillary tangles. The pathological process of AD involves the aggregation of Aβ plaques (A), tau tangles (T), and progressive neurodegeneration (N). In addition, their combined ATN framework allows diagnosis and staging of AD. Currently, clinical evaluation of AD is increasingly dependent on neuropathologically validated biomarkers Aβ and tau. Pathologically validated biomarkers for AD included Aβ and phosphorylated tau positron emission tomography (PET), cerebrospinal fluid (CSF) Aβ1-42 / Aβ1-40 ratios, CSF Aβ1-42, total tau (t-tau), and Thr181 phosphorylated tau (p-tau181) concentrations. Neuroimaging and CSF biomarkers are approved by the U.S. Food and Drug Administration for early diagnosis of AD, but their high cost, limited accessibility, and invasiveness limit their use as first-line AD diagnostic tools. The use of blood-based biomarkers can enable low-cost, widely available and non-invasive early diagnosis of AD. Therefore, screening for AD through blood tests will be an important step towards early intervention and reducing the burden on society.
A growing body of evidence supports the use of blood biomarkers for early minimally invasive detection and differential diagnosis of AD. Plasma Aβ1-42, p-tau231, p-tau181, and p-tau217 have been reported to be positively correlated with CSF homologs and significantly altered in preclinical AD. Notably, levels of Aβ1-42, t-tau, and p-tau181 in blood neuron-derived exosomes have been shown to distinguish between individuals clinically diagnosed with AD and healthy individuals or those with other types of dementia. Blood exosomes are extracellular vesicles secreted by living cells into the circulating blood. They are important mediators of intercellular communication and can reflect the physiological and disease state of the brain by transferring their proteins, lipids and nucleic acid molecules. Zheng et al. found that exosomes injected into AD mice were enriched around Aβ plaques in the brain, suggesting that exosomes were involved in plaque formation. Therefore, the authors speculate that blood exosomes are important players in the pathogenesis of AD and have potential as diagnostic markers for AD.
The use of different analytical strategies for blood exosomes has been widely reported, such as microfluidic techniques, mass spectrometry, enzyme-linked immunosorbent assay (ELISA), and supersensitive single molecule array (Simoa). Although plasma neuronal exosomes have been reported as biomarkers for AD, the lack of specific isolation and extraction methods has limited the basic research and clinical application of neuronal exosomes. The technology of blood exosome separation is becoming more and more mature, but there are no reports on AD diagnosis based on the detection of AD core biomarkers in blood exosomes. This may be due to the heterogeneity of blood exosomes and the difficulty in detecting biomarkers in blood exosomes with high sensitivity.
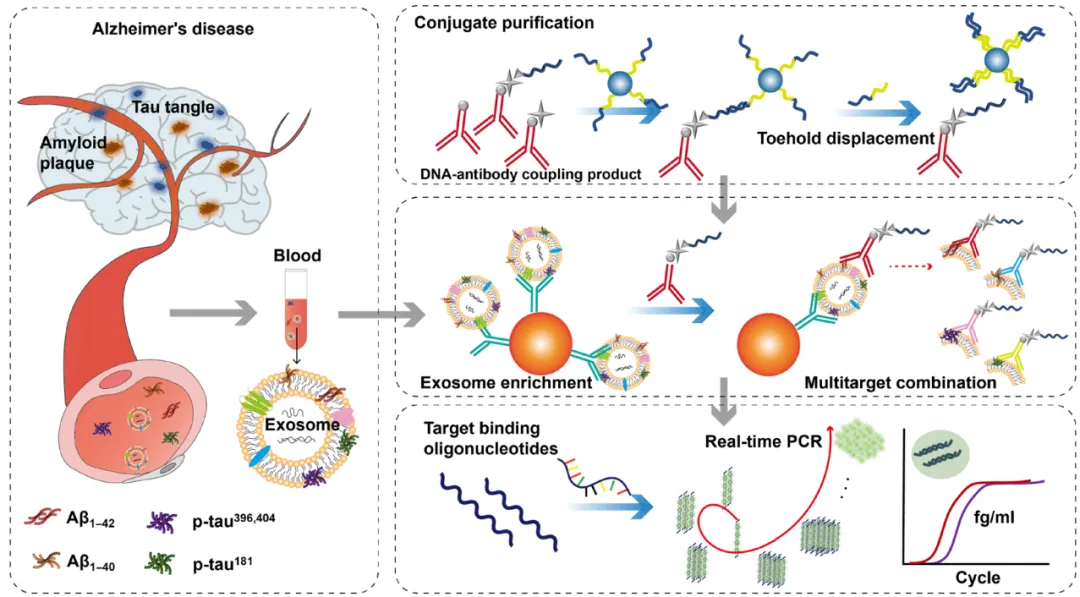 Schematic diagram of iMEP detecting Biomarkers of blood exosomes (Credit: Science Advances)
Schematic diagram of iMEP detecting Biomarkers of blood exosomes (Credit: Science Advances)
The study describes A sensitive assay platform, called immunomagnetic exosomal polymerase chain reaction (iMEP), for rapid detection of core Alzheimer's disease biomarkers (Aβ1-42, Aβ1-40, p-tau396,404, and p-tau181). The platform can enrich blood exosomes and sensitively detect multiple blood biomarkers. The iMEP system integrates immunomagnetic enrichment of blood exosomes, immunorecognition of exosomal biomarkers, and real-time polymerase chain reaction.
In this study, the DNA affinity pull-down technique mediated by toehold substitution was applied to purify the DNA-antibody conjugations of enriched exosome binding antibodies. Due to the specificity of sandwich ELISA and the enhanced binding of PCR signals, DNA conjugations of iMEP corresponding to enriched exosome binding antibodies can reliably detect target analytes at concentrations as low as 10 fg/ml. The method can selectively identify various Alzheimer's disease-related biomarkers in blood exosomes and distinguish patients with clinically diagnosed AD from healthy individuals by estimating AD biomarker levels in blood exosomes.
Original link:https://www.science.org/doi/10.1126/sciadv.abm3088