
Source: BioArt
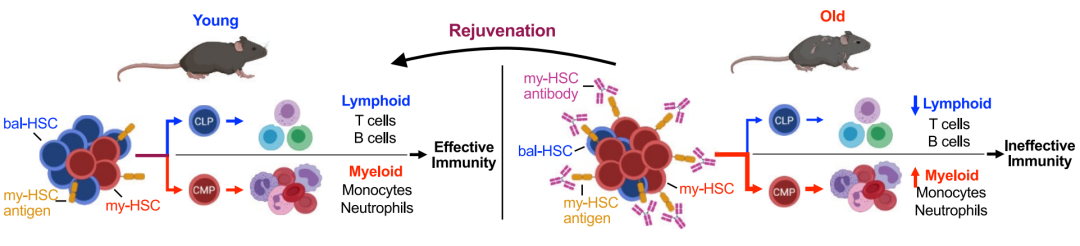
Immune system aging is characterized by reduced lymphocyte production and adaptive immune responses, and increased inflammation and myeloid pathology. Self-renewal and age-related changes in haematopoietic stem cell populations (HSCs) underlie these phenomena. A single HSCS can generate all blood cells and self-renew to maintain a stem cell pool throughout the life cycle. HSCS exhibit functional heterogeneity and may differ in their contributions to lymphoid and myeloid cell lineages. In youth, the HSC (bal-HSC) that provides a balanced output of lymphoid and myeloid cells (bal-HSC) is better than the HSC (myeloid-biased output, bal-HSC) that provides a balanced output of lymphoid and myeloid cells (BAL-HSC). my-HSC dominates, thus promoting lymphocyte production needed to initiate an adaptive immune response, while limiting the production of myeloid cells and limiting the inflammatory response. Aging is associated with an increased proportion of my-HSC, resulting in reduced lymphocyte production and increased myeloid cell production. This results in a variety of pathologic features in the elderly, including reduced adaptive immunity, inflammation, and several myeloid cell related diseases. The origin of these two subsets and the underlying mechanisms of mutual transformation remain unclear. If they are independent subsets, it may be possible to reverse the aging phenotype by eliminating MY-Hscs in older mice.
On March 27, 2024, Irving L. Weissman's team from Stanford University published a paper in Nature entitled Depleting myeloid-biased haematopoietic stem cells rejuvenates aged immunity The article. The authors used MY-HSC-specific surface marker CD150 for cell depletion, which can reduce senescence phenotype, improve lymphocyte production, and increase the body's resistance to virus.
The authors sought to determine whether it was possible to reverse age-related immune decline by restoring bal-HSC through antibody-mediated depletion of my-HSC. First authors need to identify the specific surface antigen on MY-Hscs. The authors detected and validated a set of cell surface antigens. Previous studies have found that mouse HSC (Lin−KIT+SCA1+FLT3− CD34−CD150+) can be classified as my-HSC or bal-HSC according to the expression level of CD150 (encoded by Slamf1). my-HSC is CD150-high and bal-HSC is CD150-low. In addition to CD150, several markers of HSC with myeloid bias have been described. In order to determine the best target, the authors conducted a systematic analysis of these markers. The authors found that CD150, CD41, CD62p and NEO1 were significantly enriched on the surface of my-HSC cells, and quantitative analysis by the authors found that CD150 was the most suitable target.
Next, the authors determined whether MY-Hscs in the body could be consumed by targeting these MY-Hscs antigens. To determine the effect of targeting CD150, the researchers tested the ability of anti-CD150 antibodies to deplete MY-Hscs in the body. The authors administered rat IgG2b anti-CD150 antibodies to adult mice (6-7 months of age) and assessed bone marrow about 1 week later. The results showed that the antibodies targeting CD150 were sufficient to exhaust most of the MY-Hscs in the body. The authors then examined the effects of my-HSC depletion, which showed that my-HSC depletion could rebalance progenitor cells. It was found that my-HSC depletion can increase lymphocyte production in aging mice, and the selectivity of naive T cells and mature B cells increases in aging mice. Phenotypic detection showed that my-HSC depletion could reduce the aging phenotype and reduce the expression of inflammatory markers. Analyzing the functional effects, the authors found that my-HSC depletion can improve the body's ability to resist viral infection. Finally, the authors detected the expression of markers in human HSC cell subsets, and found that CD150, CD62p and NEO1 were also enriched in HSC subsets, which laid a foundation for further experiments in human cells.
In conclusion, in order to promote lymphocyte generation and limit the inflammatory response driven by myeloid cells, the authors identified different cell surface markers of my-HSC and bal-HSC, targeted and exhausted my-HSC in vivo, and verified that their depletion can reduce the aging phenotype, promote the increase of lymphocytes, and reduce the inflammatory response related to myeloid cells. Improve your ability to fight viral infections.
The original link:https://doi.org/10.1038/s41586-024-07238-x