
On April 1, 2024, Nature Communications, a subsidiary of the world's leading journal Nature, published the stunning results of a "Phase I clinical trial of adipose-derived mesenchymal stem cells for the treatment of traumatic spinal cord injury." The sensory and motor functions of 7 enrolled patients improved after receiving stem cell therapy, and no serious adverse reactions were observed during follow-up, which not only marked a major discovery in the treatment of spinal cord injury, but also verified the safety and feasibility of using adipose stem cells to treat spinal cord injury.

Phase 1 results of adipose-derived mesenchymal stem cells are stunning, bringing "light at the end of the tunnel" for spinal cord injury patients
This Phase I clinical trial of adiply-derived Mesenchymal Stem Cells (AD-MSC) for the treatment of Traumatic Spinal cord Injury (NCT03308565) was reviewed by the U.S. Food and Drug Administration (FDA) and the Mayo Clinic Institutional Review Board.
PART 01 Enrolled patient
A total of 10 adult patients with spinal cord trauma (6 with neck injury and 4 with chest injury) were enrolled in this study and were rated as grade A (8 cases) or Grade B (2 cases) by the American Spinal Injury Association Injury Scale (AIS) at the time of injury. All patients were enrolled within 12 months of injury (range 2-12 months).
PART 02 Therapeutic process
After these patients were enrolled, firstly, fat collection was performed; Subsequently, AD-MSC was isolated and expanded, and the stem cells were expanded to a dose of 100 million cells. Finally, under fluoroscopic guidance, the prepared stem cells were injected into the patient's lumbar spine (i.e., "subarachnoid injection"), and the patient was hospitalized for 24 hours after the injection. The specific treatment process is shown in the following figure:
Figure 1. An example of the operation of AD-MSC for spinal cord injury
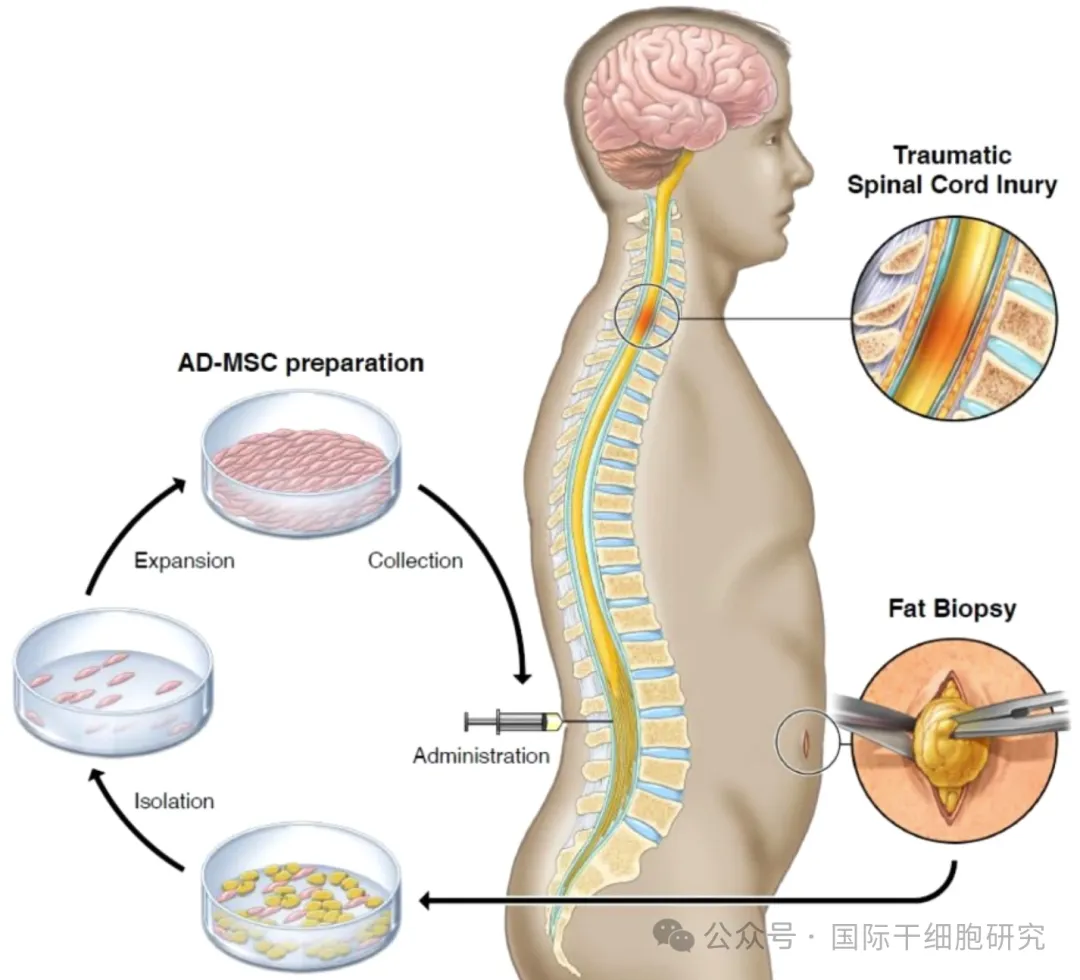
Note: The above figure shows the whole process of adipose tissue collection, AD-MSC isolation and expansion, and stem cell transfusion
PART 03 Result analysis
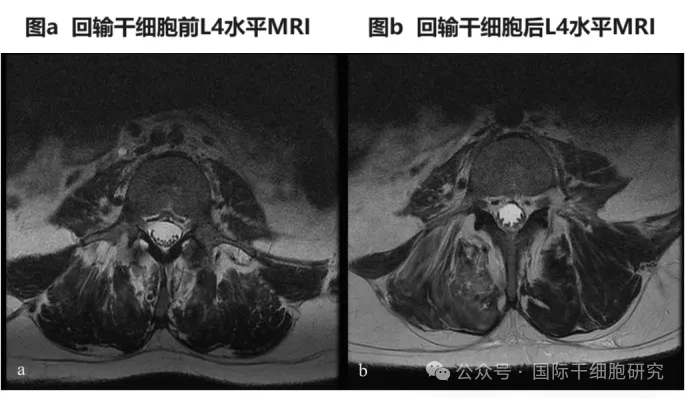
1、MRI showed a slight increase in cauda equina root clusters in 8 patients, improved spinal evoked potential (SSEP) in 3 patients, and normal SSEP in 1 patient during follow-up. MRI comparison of patient 6 before and after reinfusion of adipose-derived mesenchymal stem cells (AD-MSC) showed a slight increase in caudal nerve root clusters at the L4-S1 level (see the figure below).
Figure 2. Comparison of MRI before and after AD-MSC transfusion in patient No. 6
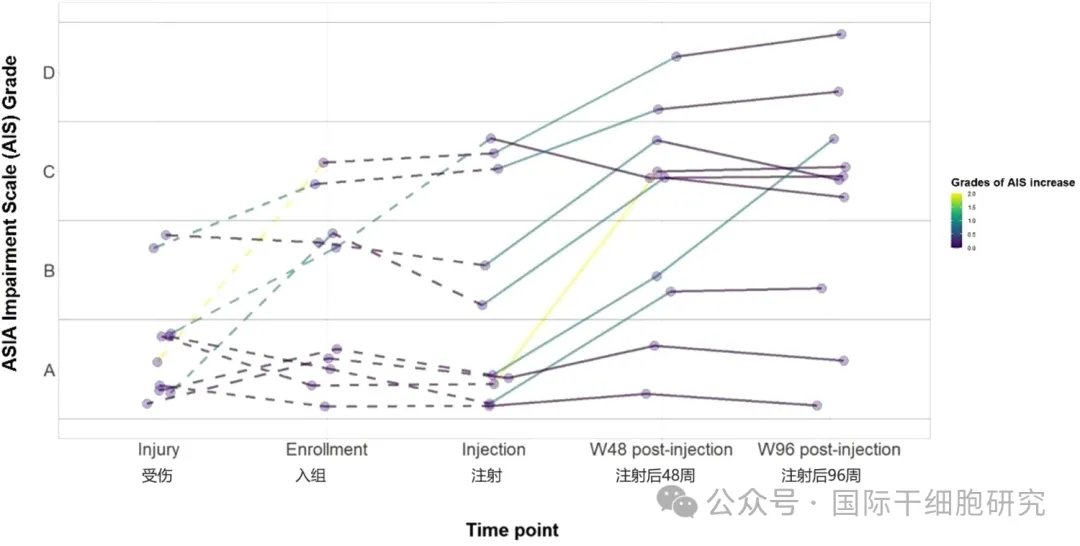
2、American Spinal Injury Association Injury Scale (AIS) rating
At the last follow-up, seven patients showed improvement in AIS grade and at least one level of motor and sensory function after injection; The other two patients experienced at least one level of improvement in sensation (see figure below).
Figure 3. AIS grade at injury, enrollment, injection, and final follow-up
3、Cerebrospinal fluid parameters
Seven of the nine evaluable patients showed elevated levels of VEGF (vascular endothelial growth factor) after injection of AD-MSC.
In conclusion, adipose-derived mesenchymal stem cells (AD-MSC) have the advantages of easy access, pluripotency and availability, and have broad application potential in the treatment of spinal cord injury.
What do you know about spinal cord injury
PART 01 The anatomy of the spinal cord
The spine surrounds and protects the spinal cord and is made up of 33 bone rings (vertebrae), cartilage pads (intervertebral discs), and "foramen" (narrow Spaces).
The spinal cord is a soft cylinder made up of blood vessels, tightly packed cells (nerve cells and glial cells), and nerve fibers (called axons) that transmit nerve signals. Like the brain, the spinal cord is protected by three layers of tissue and surrounded by cerebrospinal fluid (CSF), which acts as a cushion against shock or injury.
Nerve bundles extend from the brain and cross the spinal cord at all levels from a network of nerve fibers throughout the body, whose purpose is to send sensory and motor information. The spinal cord is responsible for sending and receiving information between the brain and the rest of the body, and damage to the spinal cord can disrupt these signals, resulting in a range of motor or sensory impairments.
PART 02 The harm of spinal cord injury
Spinal cord injury is caused by trauma (such as traffic accidents, sports injuries, falls) or lesions (such as epidural hematoma, inflammation) and other factors, resulting in the injury of the spinal cord and its function, patients may lose motor or sensory function, and even have the risk of paralysis in severe cases, seriously affecting physical and mental health, and will bring heavy economic burden and nursing pressure to the family. The pathological manifestations of spinal cord injury vary with different segments.
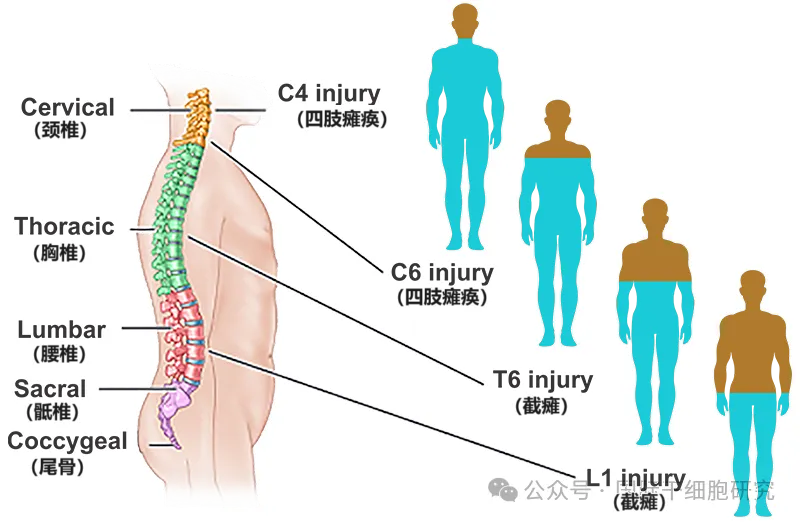
1, cervical nerve (C1~C7) : control the neck, back of the head, shoulders, hands and arms and other signals, once the injury of these parts of the sensory or motor function will be affected.
2, thoracic nerve (T1~T12) : control the chest muscles, some muscles in the back, organs and other signals.
3, lumbar nerve (L1~L5) : control the lower abdomen, back, buttocks, external reproductive organs, legs and other signals.
4, Sacrospinal nerve (S1-S5) : control the thigh, lower leg, foot, around the anus and most of the external genital organs and other signals (see the figure below).
The principle of stem cell therapy for spinal cord injury
Stem cells have the potential to promote nerve recovery, regenerate neural networks, and repair damage. At present, a large number of preclinical and clinical research data show that mesenchymal stem cells (MSC) can play a therapeutic role through various pathways such as immune regulation, endogenous neurogenesis, angiogenesis, and neuronal plasticity. The main reason why stem cells can treat spinal cord injury is due to the following mechanisms:
PART 01 Repair nerve damage
Spinal cord injury can lead to the death of glial cells and neurons, and progressive demyelination can also denature axonal fibers, thus affecting the normal transmission of axonal glial signals.
On the one hand, stem cells can provide nutrients to support endogenous cells and promote the recovery of spinal cord injury. On the other hand, it has the ability to differentiate into lineages of glial cells (including astrocytes, oligodendrocytes, and neurons), thereby inhibiting myelination of naked axons, inhibiting gliosis, and ultimately promoting better neurite growth.
PART 02 Immunomodulation
Stem cells can play a role in immune regulation by secreting transforming proliferation factor-β (TGF-β) and interleukin-10 (IL-10), so as to help reduce neuroinflammation, improve surrounding tissue damage, and inhibit the occurrence of secondary spinal cord injury.
PART 03 Paracrine characteristic
Stem cells have significant paracrine and autocrine activities, and can promote the proliferation and regeneration of residual neurons by secreting a variety of neurotrophic factors (such as glial neurotrophic factor, brain-derived neurotrophic factor, and nerve growth factor), and also help protect surviving neurons.
PART 04 Other factors
Stem cells can also be involved in tissue regeneration and functional recovery, and further promote blood vessel and nerve regeneration, blood-spinal barrier repair, thereby improving spinal cord injury. For example, AD-MSC (adipose mesenchymal stem cells) mentioned above, compared with other adult stem cells, such stem cells have excellent angiogenesis and nerve regeneration capabilities, but also have the advantages of pluripotency, availability and easy access, so it has broad application potential in the field of spinal cord injury treatment.
Overview of clinical application and research status of stem cell therapy for spinal cord injury at home and abroad
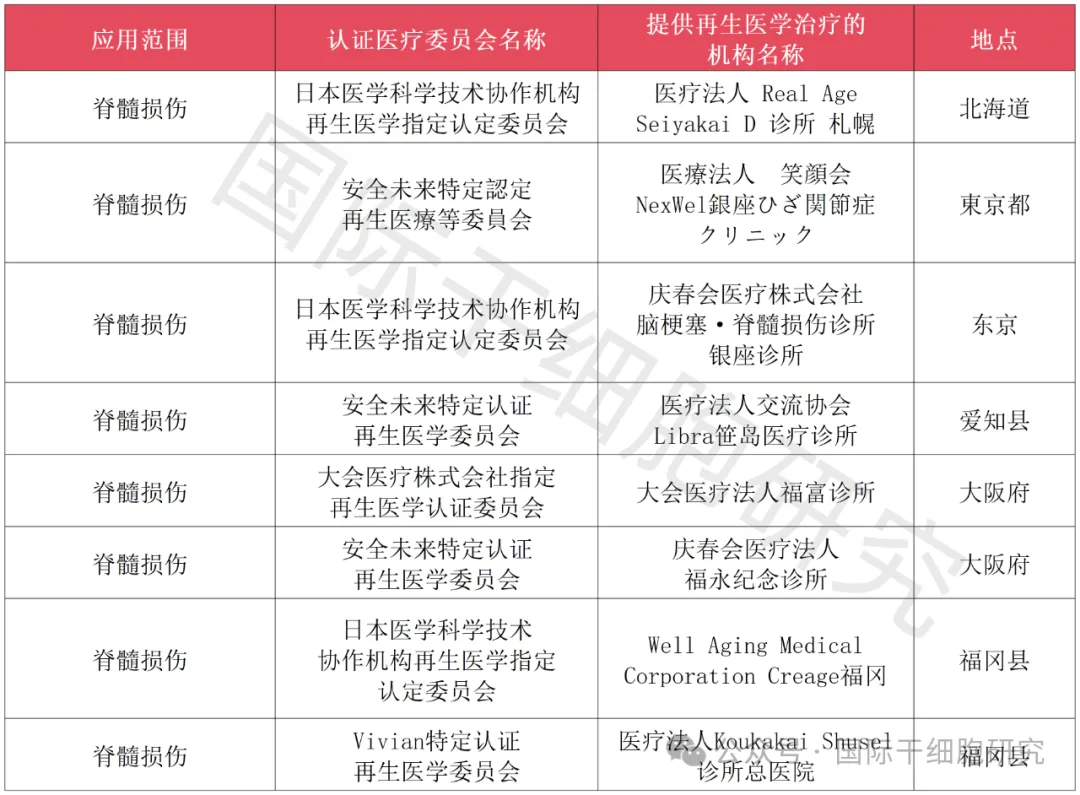
1、The Japanese Ministry of Health, Labour and Welfare applied for approval of autologous adipose-derived stem cells for the treatment of spinal cord injury project
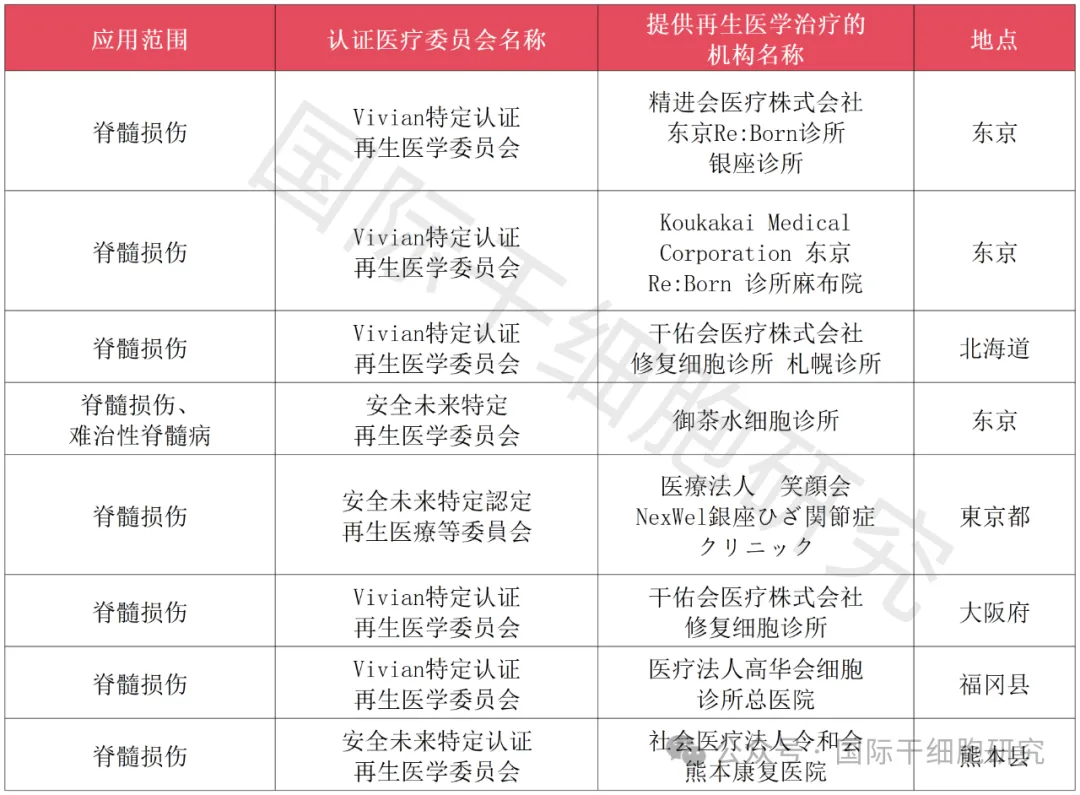
2、The Japanese Ministry of Health, Labour and Welfare applied for approval of bone marrow mesenchymal stem cells for the treatment of spinal cord injury project
3、Summary of MSC drugs for Spinal cord Injury in China, Implied Authorization for Clinical Trials (IND)

Small series message
Spinal cord injury will not only seriously affect the physical health and quality of life of patients, but also bring a heavy burden to patients and their families. At present, traditional treatment is mainly to improve symptoms, and it is difficult to regenerate damaged neurons, which is one of the reasons why spinal cord injury is difficult to treat.
However, it is gratifying that with the development of regenerative medicine in recent years, stem cells with neuroprotective and regenerative effects have opened a new chapter for the treatment of spinal cord injury. The phase 1 clinical study of "adipose stem cells for Spinal cord Injury" reported in the sub-issue of the world-renowned journal Nature also achieved positive results, bringing a ray of light to the intractable disease of spinal cord injury!
However, it is worth noting that all patients included in this study received stem cell therapy within 12 months after injury. As for the effect of stem cell therapy on old spinal cord injury, more clinical studies are needed to verify it.