
Source:iNature
Understanding of early human development has been hampered by a lack of reference datasets from natural embryos, especially those with spatial information about key stages such as gastrula formation.
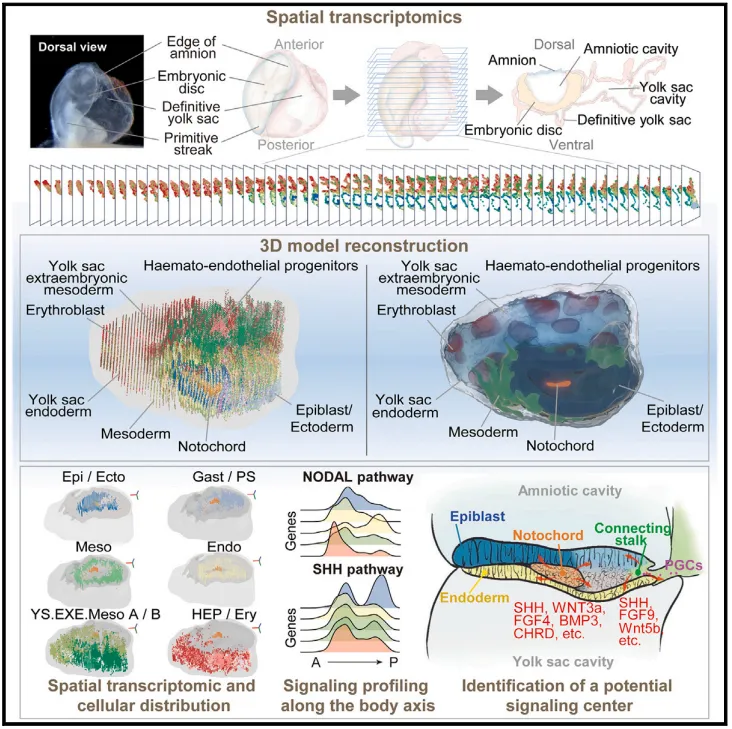
On April 23, 2024, Leqian Yu and Jingtao Guo, researchers from the Institute of Zoology of the Chinese Academy of Sciences and the Beijing Institute of Stem Cell and Regenerative Medicine, Professor Yulei Wei from China Agricultural University and Dr. Wang Xiaoyan from the Institute of Zoology of the Chinese Academy of Sciences, wrote in a joint statement entitled "3D reconstruction of a gastrulating ulating. "human embryo" research paper, which performed high-resolution spatial transcriptomic analysis of 38,562 points on 62 cross-sections of a complete Carnegie stage (CS) 8 human embryo.
From this spatial transcriptome dataset, the study constructed a 3D model of the CS8 embryo, in which a range of cell subtypes were identified along the anteroposternal, posteroposternal and dorsoventral axes of the embryo, based on gene expression patterns and location information. The study further describes the lineage locus of the embryo and extra-embryonic tissues, as well as the associated regulation and regionalization of signaling centers, and the signaling activity that supports lineage progression and tissue patterns during gastrula formation. The findings of this study provide insights into gastrula formation and postgastrula development in human embryos.

The formation of gastrula is the transformation of the double disk of embryo within the blastula into a complex multi-dimensional structure, the gastrula. This complex event laid the foundation for different cell lineages and spatial patterns. Human gastrulation occurs on days 14 to 21 of the embryo after fertilization (E14-21), corresponding to Carnegie stages (CS) 7 to 9. During this time, the ectoderm (Epi) is allocated to the precursors and derivatives of the primary germ layer and the extra-embryonic tissue. In addition to determining cell lineages, the establishment of a body axis is essential for coordinating patterns of migration, localization, and differentiation of cells.
Dynamic signal centers, such as notochords, regulate the establishment of the body's axis and guide the development of cells to their designated destinations. This disruption of coordination can lead to serious congenital abnormalities. However, the study of human embryogenesis faces unique challenges, as the scarcity of early human embryonic material in the womb limits direct research. As a result, researchers' understanding of human gastrula relies heavily on data from other sources. Animals, such as mice, are the main source of knowledge about gastrum formation.
In particular, the application of single-cell RNA sequencing (scRNA-seq) has provided an increasingly detailed view of developmental gene expression and developmental pathways in each lineage. However, the uniqueness of primate embryogenesis in terms of anatomical structure, developmental timing, and lineage fate decisions makes it difficult to understand primate embryos solely by studying model animals. Methods developed in recent years for in vitro culture of primate blastocysts and embryonic models based on pluripotent stem cells have allowed researchers to study ontogenesis of embryonic lineages outside the womb and have revealed several developmental milestones in early primate embryos. However, due to the lack of relevant literature, there are some difficulties in whether these stem cell-derived embryonic models can faithfully reproduce embryonic development.
 Model diagram (Credit: Cell)
Model diagram (Credit: Cell)
For primate embryos in utero, recent transcriptomic characterization of early gastrula developing human embryos (CS7) and cynomophobe monkey gastrula developing embryos has provided important insights into primate gastrula development. Despite these advances, these datasets lack spatial positioning information, which makes it challenging to accurately annotate cell subtypes. The question of how cell fate is determined in different locations during gastrulation, and how the various subtypes of cells coordinate and develop along the body axis, remains fragmented. In recent years, spatial analysis of marmoset embryos has been carried out during the CS5 to CS7 stages after implantation. This study used laser capture microanatomy in combination with Smart-seq2 to uncover important developmental events.
The study performed a continuous cross-sectional view of 62 sections of a complete gastrula CS8 embryo, which allowed the researchers to combine the spatial transcriptomes of all sections from the front to the back side, allowing for a complete 3D reconstruction of the embryo. This dataset provides an opportunity to study the basic cellular and molecular characteristics of human gastrula formation events and provides valuable information for the development of advanced stem cell-derived models of human embryos.
Original link:https://www.cell.com/cell/abstract/S0092-8674(24)00357-X#%20