
Epigenetic reprogramming resets the epigenetic memory of parents and differentiates primordial germ cells (PGCs) into premitotic spermatogonia or oogonia to ensure sex-differentiated germ cell development and totipotency. Reconstructing human epigenetic reprogramming in vitro remains a fundamental challenge.

Recently, the Mitinori Saitou team of the iPS Research Institute of Kyoto University published an article entitled "In vitro reconstitution of epigenetic reprogramming in the human germ line" in the journal nature. In this study, human-like PGC (hPGCLCs) derived from induced pluripotent stem cells (iPSC) were epigenetically reprogrammed and differentiated into premitotic spermatogonia or oogonia, accompanied by extensive expansion (about 1010 times).
It is worth noting that bone morphogenetic protein (BMP) signaling is a key driver of these processes: BMP-driven hPGCLC differentiation involves weakening the mitogen-activated protein kinase / extracellular regulated protein kinase (MAPK/ERK) pathway and the neogenesis and maintenance of DNA methyltransferase (DNMT) activity, which may promote replication-coupled passive DNA demethylation. On the other hand, TET-1-deficient hPGCLCs (an active DNA demethylase rich in human germ cells) differentiates into extraembryonic cells, including amniotic membrane, and has the de-inhibition of key gene promoters; these cells fail to fully activate genes critical to spermatogenesis and oogenesis, and their promoters remain methylated.
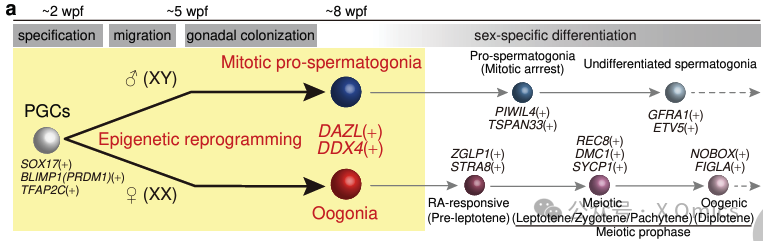
Germ cells give totipotency and ensure heredity and evolution. Human primordial germ cells (hPGCs) are thought to be specific in the amniotic membrane or mesoderm after early embryo implantation at about 12-16 days (2 weeks after fertilization). They migrate through the yolk sac and hindgut endoderm and begin to colonize the reproductive ridge about 5-6 weeks later. During this period, hPGCs initiates epigenetic reprogramming to reset the epigenetic memory of parents through genome-wide DNA demethylation (5-methylcytosine demethylation) and histone modification remodeling. About 7-8 weeks later, hPGCs showed the completion of reprogramming and differentiated into premitotic spermatogonia or oogonia, the precursors of spermatogonia or oocytes, respectively.
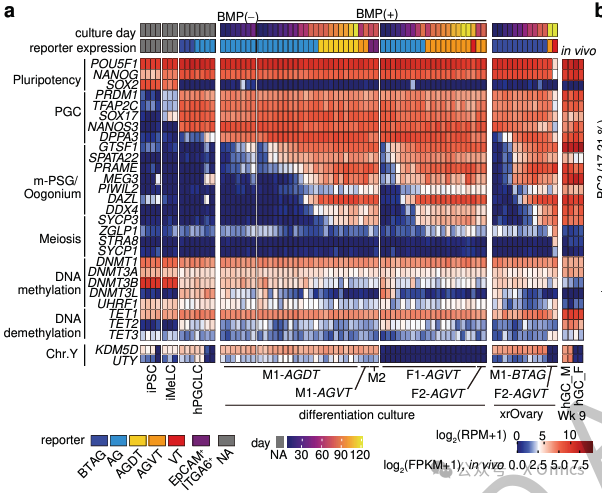
The discovery that BMP signal stabilizes the fate of germ cells is similar to the role of BMP signal in maintaining self-renewal of embryonic stem cells by blocking differentiation. Considering that inhibition of MAPK/ERK signal plays a key role in inducing primitive pluripotency associated with DNMTs inhibition and DNA methyl reprogramming, a similar mechanism may also be applicable to hPGCLC differentiation. BMP signal and its correlation in PGC epigenetic reprogramming of other species are worthy of further study.
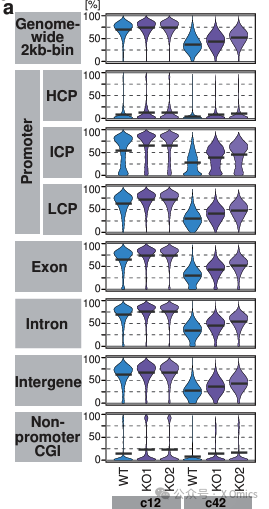
In mice, Tet1 itself is not necessary for genome-wide DNA demethylation, but is essential for maintaining demethylation of key genes in spermatogenesis and oogenesis as well as imprinted DMR. In order to better understand the epigenetic reprogramming of TET function in humans, it will be necessary to evaluate catalytic mutants while keeping their transcriptional inhibitory activity intact.
This study clarified the framework of human epigenetic reprogramming, made fundamental progress for human biology, and through the production of a large number of premitotic spermatogonia and oogonia, it represents a milestone in the study of human gametogenesis in vitro and its potential transformation in reproductive medicine.
Original link: https://doi.org/10.1038/s41586-024-07526-6