
On May 20, 2024, there was a breakthrough in the field of stem cell therapy. The Swedish research team treated patients with Parkinson's disease based on stem cell technology with excellent results. Once again show that stem cell therapy is promising in the future, boosting industry confidence.
Swedish Parkinson's disease patient Thomas Matterson (Thomas Mattson) received stem cell-derived nerve cell STEM-PD transplantation in 2023, becoming the first Parkinson's patient in the world to undergo brain cell implantation in 7 million laboratories.
The STEM-PD trial, conducted by Lund University in Sweden and sponsored by established biopharmaceutical giant Novo Nord and agencies in Sweden and the European Union, is testing a new stem cell therapy that aims to replace missing dopamine nerve cells in the brains of patients with Parkinson's disease with healthy cells made from stem cells.

Recently, Professor Wang Renzhi, Professor Bao Xinjie, and Professor Wan Xinhua, Department of Neurology, Peking Union Medical College Hospital conducted a phase I clinical study on the safety and preliminary efficacy of intranasal transplantation of human neural stem cells (ANGE-S003) in the treatment of Parkinson's disease. The relevant research results were officially published online in Journal of Neurology, Neurosurgery& Psychiatry (IF:11.1, Q1/TOP, Chinese Academy of Sciences) on May 10, 2024.
This is also the first clinical study of transnasal transplantation of human neural stem cells in the treatment of Parkinson's disease.
Parkinson's disease (PD), also known as "tremor paralysis", is a degenerative disease of the central nervous system, mainly caused by the progressive loss of dopaminergic neurons in the substantia nigra. At present, the treatment of Parkinson's disease is mainly focused on drugs and surgery. Unfortunately, these methods are not effective for every patient, and more importantly, traditional treatments cannot reverse neurodegeneration or increase the number of dopaminergic nerve cells.
Traditional treatment can only relieve symptoms, not cure PD.
The researchers turned their attention to the neural stem cells with good potential for self-renewal and the ability to differentiate into neurons, astrocytes and oligodendrocytes and improve the cellular microenvironment, and believe that neural stem cells (NSC) provide a new idea and method for the treatment of Parkinson's disease, and have made some progress.
In recent years, neural stem cells have a variety of therapeutic potential in the treatment of central nervous system diseases, and can repair nerve injury in several ways.
1. The transplanted stem cells can migrate to the injured nerve site, replace the dead or injured nerve cells through cell substitution, and repair the damaged neural network.
2. Transplanted stem cells can secrete a large number of active factors and nutritional factors, activate nerve cells, promote the regeneration and reconstruction of new cells, and secrete angiogenic factors to promote angiogenesis in the lesion site.
3. Can carry out immune regulation, stem cells can regulate the number of immune cells to reach the pathological site and secrete different levels of cytokines to influence each other, playing a protective role in anti-inflammation.
这是一项为期12个月的单中心、开放标签、剂量递增的1期研究(该I期临床研究起自2019年9月至2021年3月),对18名晚期PD患者进行四次鼻内移植,移植剂量为3个剂量中的1个:150万、500万或1500万ANGE-S003人类神经干细胞,以评估其安全性和有效性。
Eighteen patients with Parkinson's disease were divided into three dose groups (1.5 million, 5 million or 15 million human neural stem cells). Six subjects in each group received transnasal transplantation of neural stem cells once a week for 4 times. A 12-month follow-up evaluation was completed at the end of treatment.
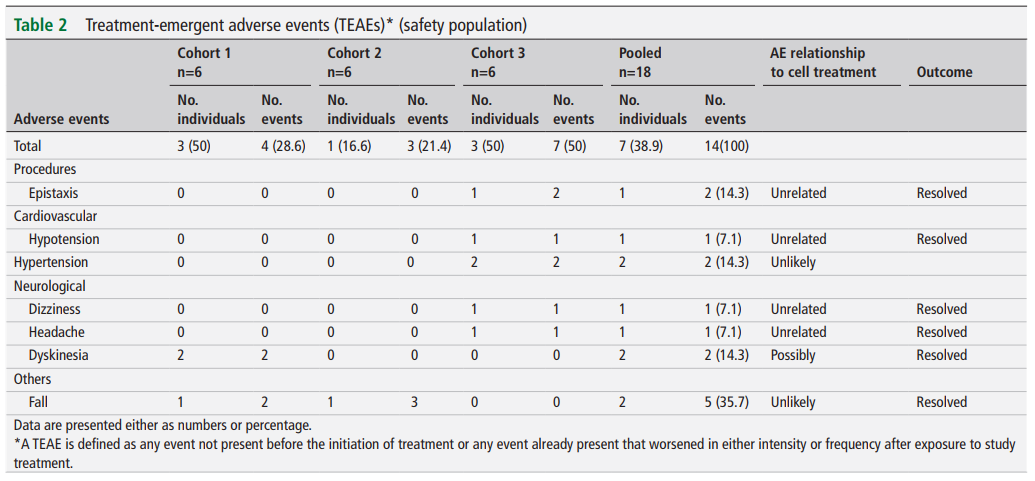
The main results showed that during the 12-month follow-up period after treatment, a total of 14 adverse events occurred in 7 subjects, and no serious adverse events related to ANGE-S003 neural stem cells occurred. The safety examination did not find any clinically meaningful safety problems. Brain magnetic resonance imaging showed no signs of tumor formation or abnormality.
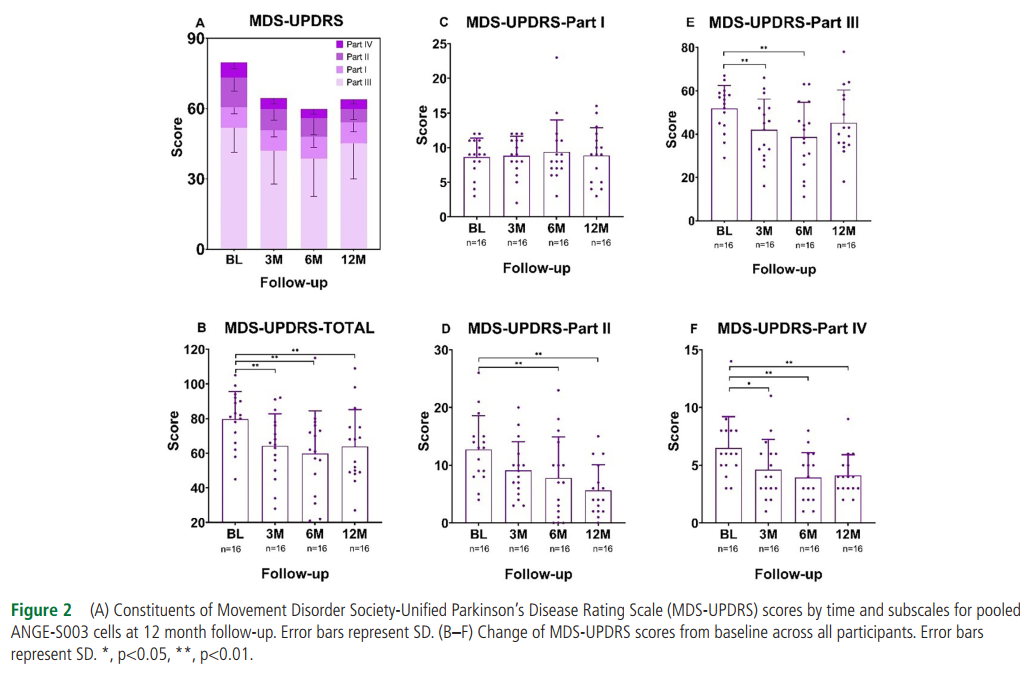
The secondary outcome showed that among the 16 subjects with 12-month MDS-UPDRS data, the total MDS-UPDRS score improved significantly at all time points (P < 0.001), and the improvement began from the 3rd month to the 12th month. Among them, the improvement in the 6th month after treatment was the largest, with an average decrease of 19.9 points (95% confidence interval, 9.6 to 30.3%, P < 0.001).
There was no correlation between the improvement of clinical outcome indicators and the level of cell dose.
Conclusion: in this clinical study of transnasal neural stem cell transplantation for patients with Parkinson's disease for the first time, the results showed that transnasal neural stem cell transplantation was safe and reliable, and the subjects showed good tolerance. With the passage of time, the clinical symptoms of the subjects improved, and the efficacy of neural stem cells reached the peak at 6 months after treatment. Intranasal transplantation of neural stem cells represents a new approach to the treatment of PD. It is necessary to conduct a larger, longer-term, randomized controlled phase 2 trial for further study.
Reference:
Jiang S, Wang H, Yang C, et al,Phase 1 study of safety and preliminary efficacy of intranasal transplantation of human neural stem cells (ANGE-S003) in Parkinson’s disease
Journal of Neurology, Neurosurgery & Psychiatry Published Online First: 09 May 2024. doi: 10.1136/jnnp-2023-332921