
The process of inducing reprogramming of induced pluripotent stem cells (iPSC) involves several key events, including somatic gene shutdown, pluripotent gene activation, mesenchymal epithelial transition (MET), metabolic reprogramming, and epigenetic remodeling. Although these events interact intricately and affect each other, the specific factors that regulate the reprogramming network are unclear. Double Homeobox (Dux) is a factor known to promote totipotency during the transition from embryonic stem cells (ESC) to 2C-like embryonic stem cells (2CLC), but has not been extensively studied in the context of iPSC reprogramming. Lactation modification of the 18th lysine of histone (H3K18la) is affected by glycolytic metabolic products. It is a transcriptional activating modification that can regulate gene expression and affect cell fate at the chromosomal level. It is a connection between genetic information and environmental factors.
On March 15, 2024, Professor Lei Lei's research team from the Department of Histology and Embryology, School of Basic Medicine, Harbin Medical University published a research paper titled "Dux activates metalloism-lactation-MET network during early iPSC reprogramming with Brg1 as the histone lactationreader" online in the journal Nucleic Acids Research. In this study, it was proved that Dux-H3K18la-MET regulates the network during iPSC reprogramming.
Dux affects H3K18la levels by affecting metabolites and regulating histone modifying enzymes. In addition, the study's proteomic analysis based on H3K18la immunoprecipitation experiments revealed specific recruitment of Brg1 during reprogramming. Both H3K18la and Brg1 are enriched in gene promoters associated with pluripotency and epithelial connectivity. Its role as a reader of histone lactation has been demonstrated through protein interactions and molecular docking. This research will help to further understand and improve the mechanism of histone lactation modification regulating biological processes.
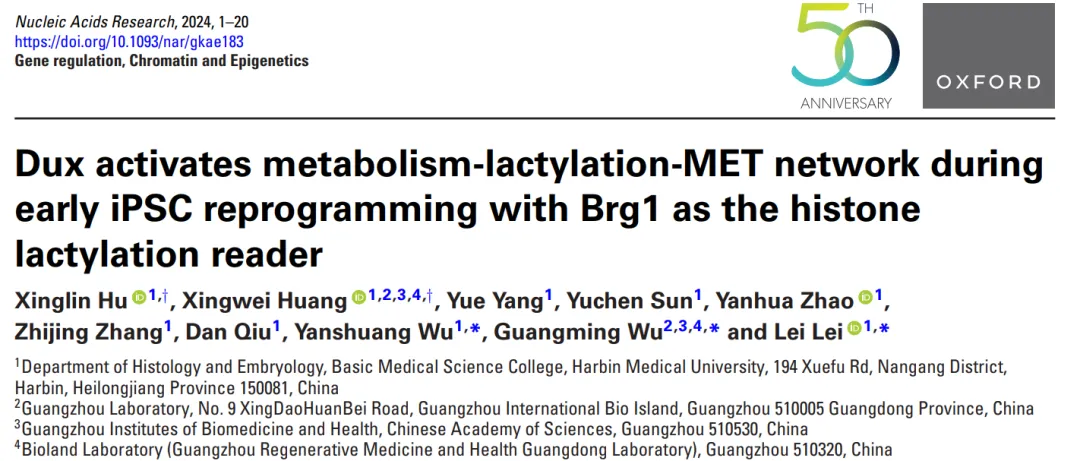
Dux's role in embryonic development has received great attention in recent years because of its ability to promote the transformation of ESCs into totipotent stem cells with developmental potential. During mouse embryonic development, Dux mRNA shows specific high transcription levels during the early 2-cell stage, which is consistent with the initiation of the first wave of zygotic genome activation ( ZGA ). Although Dux has been shown to be not indispensable for ZGA, there is evidence that it plays a crucial role in the transformation of 2CLCs. 2CLCs are a subset of ESCs that manifest as spontaneous expression of mouse 2 -cell embryo-specific genes and repetitive sequences. Compared to ESC, 2CLCs have higher levels of H3K27ac, H3K4me3, and H3K4me1 modifications. In addition, 2CLCs are more comprehensive pluripotent than ESCs because they not only contribute to all three germ layers, but also contribute to trophectoderm ( TE ) in chimera experiments. Previous reports have shown that the transformation of 2CLCs is initiated by down-regulation of Myc and Dnmt1, leading to increased Dux expression. In addition, withdrawal from 2CLCs status is initiated by Smg7/Upf1 inhibition of Dux mRNA. These findings emphasize the important role of Dux in the acquisition of totipotency.
In addition, Dux has also made important contributions to the advancement of reprogramming in somatic cell nuclear transfer ( SCNT )[2]. Dux enhances the expression of the ZGA gene in 2 -cell SCNT embryos by increasing H3K9ac and H3K27ac modifications, resulting in higher pre-and post-implantation development rates. The N-terminal of Dux protein consists of two highly conserved adjacent DNA-binding homologous domains, while the C-terminal of Dux recruits CBP/p300, which is conducive to the accumulation of histone H3 acetylation. Promoting histone H3 acetylation has previously been established as a means to improve the efficiency of iPSC reprogramming. Studies have shown that Duxbl promotes MET in muscle stem cells, an early key step in iPSC reprogramming. In addition, other characteristic genes known to promote 2CLC have also been found to enhance iPSC reprogramming by promoting H3 acylation or metabolic rearrangement. Therefore, the authors hypothesized that Dux might play a role in promoting iPSC reprogramming, especially in the initial stages of reprogramming.
The establishment of iPSCs requires the reprogramming of differentiated somatic cells to a pluripotent state. Although Yamanaka factor has the potential to reprogram all differentiated somatic cells, the successful completion of the reprogramming process requires multiple events to occur in a sequential or simultaneous manner. Therefore, a considerable number of cells cannot complete reprogramming and are trapped in the initial stage of reprogramming, resulting in reduced reprogramming efficiency. The traditional iPSCs reprogramming process involves a variety of key events that run at different levels of regulation.
At the chromatin level, many specific open sites associated with fibroblasts quickly close in the early stages of reprogramming, while many sites associated with pluripotency become open in the later stages. At the epigenetic level, there is a decrease in overall genomic methylation levels, accompanied by a gradual increase in histone H3 and H4 acetylation levels. At the transcription level, soma-related genes were significantly reduced in the first transcription wave, and then pluripotency related genes were significantly increased in the second transcription wave. At the metabolic level, there is a shift in the primary energy supply pathway from oxidative phosphorylation ( OXPHOS ), which is commonly utilized by somatic cells, to aerobic glycolysis, which is preferred by stem cells. This transition is called the OXPHOS to glycolytic transition ( OGS ). At the morphological level, cells undergo the MET process, transformation from fibroblast-like cells to epithelial-like cells. This transformation is accompanied by significant changes in gene expression, particularly cell adhesion molecules such as Cdh1, also known as E-cadherin. Therefore, the researchers speculate that there is a fundamental factor in the initial stages of reprogramming that drives stem cell-related processes in the direction of pluripotency.
Epigenetic regulation includes multiple important mechanisms, among which histone post-translational modifications ( PTM ) are of great significance. Specifically, histone acetylation modifications have been widely studied and are believed to promote transcription by offsetting positive charges on the lysine side chains or recruiting specific reader proteins. Except for Dux, academia agrees that metabolites have the ability to influence the degree of post-translational modification of histones. One of these metabolic products is lactic acid, which is the main product of glycolysis. Lactate-CoA can serve as a substrate for histone modification, thereby regulating the accessibility of DNA molecules and thus regulating gene expression [3]. Studies have shown that H3K18la plays a crucial role in regulating the distribution of transcription elements, especially in enhancers of tissue-specific genes. Many recognized histone modifying enzymes, such as p300, act as lactoyltransferases responsible for histone lactation.
When the classical HDAC1 - 3 and SIRT1 - 3 enzymes were present, a decrease in histone lysine lactogenation, especially H3K18la and H4K5la, was observed, indicating that these classical Type I HDACs can serve as effective "erasers" of lysine lactogenation in vitro. However, the identification of "readers" of lactosylation modifications has not been reported in the literature. The recognition process of histone acetylation and deacetylation involves three main domains: the bromine domain ( BD ), the double PHD finger ( DPF ) domain and the YEATS domain. The BD module is known for its conservation, binding to acetylated lysine residues on histones. Given the conservation of binding between bromodomain homologous proteins and the structural, functional and evolutionary similarities between histone lactation and histone acetylation, it is reasonable to speculate that proteins containing BD modules can also serve as histone lactosylation."reader".
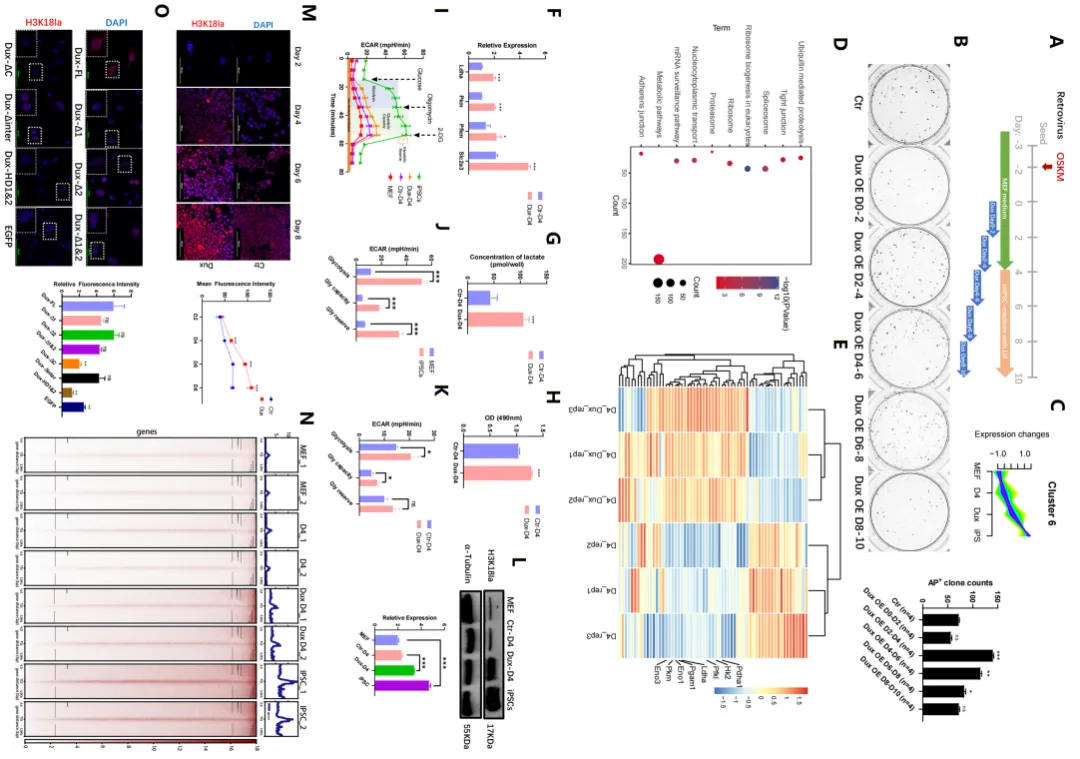
Dux promotes reprogramming efficiency of iPSCs through metabolic remodeling (Photo from Nucleic Acids Research)
Based on this, the researchers found that transient overexpression of Dux led to enhanced iPSC reprogramming efficiency by up-regulating H3K18la levels. This study established the link between OGS, histone lactation and MET through H3K18la in the initial stage of reprogramming, highlighting the key role of H3K18la in successful reprogramming. Specifically, Dux promotes an increase in H3K18la levels by promoting OGS and using its C-terminal to recruit p300. Brg1 then interacts with H3K18la, which enriches on the promoter of MET-related genes and serves as a reader for lactation to facilitate the reprogramming process.
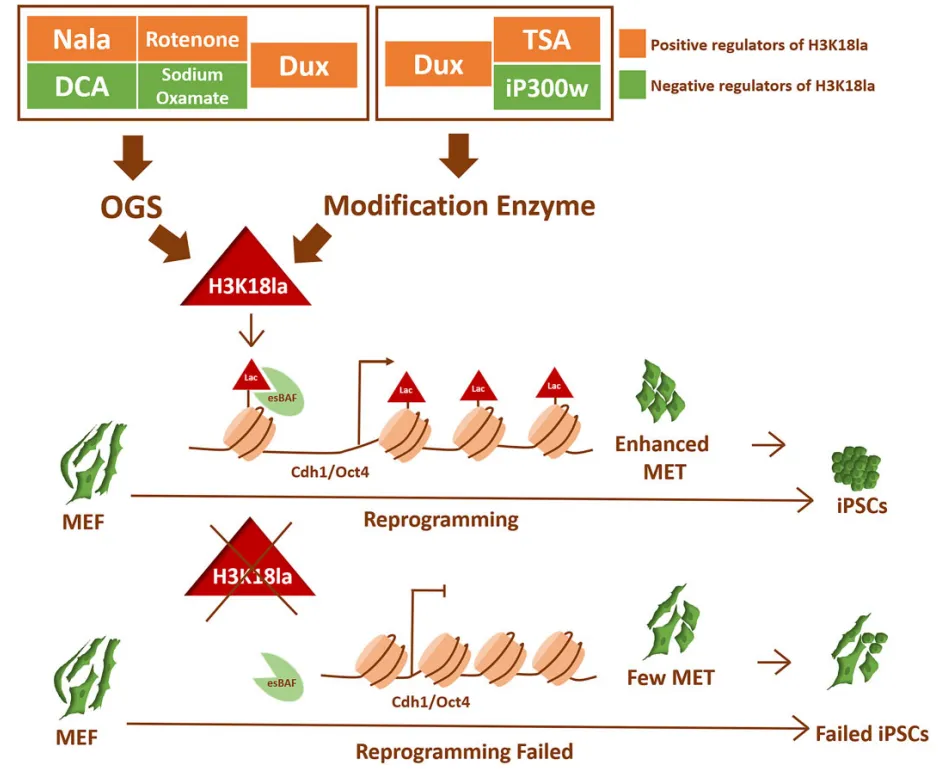
Dux promotes reprogramming efficiency of iPSCs through metabolic remodeling (Photo from Nucleic Acids Research)
Professor Lei Lei and Associate Professor Wu Yanshuang of Harbin Medical University, researcher Wu Guangming of Guangzhou Institute of Biomedicine and Health, Chinese Academy of Sciences, are co-corresponding authors of the article, and Hu Xinglin and Huang Xingwei of Harbin Medical University are co-first authors of the article.
References:
【1】Hu X, Huang X, Yang Y, Sun Y, Zhao Y, Zhang Z, Qiu D, Wu Y, Wu G, Lei L. Dux activates metabolism-lactylation-MET network during early iPSC reprogramming with Brg1 as the histone lactylation reader. Nucleic Acids Research, 2024,
【2】Huang X, Hu X, Jiang Q, Cao Q, Wu Y, Lei L. Functional study of distinct domains of Dux in improving mouse SCNT embryonic development dagger. Biol Reprod, 2021
【3】Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, Ding J, Czyz D, Hu R, Ye Z, He M, Zheng YG, Shuman HA, Dai L, Ren B, Roeder RG, Becker L, Zhao Y.Metabolic regulation of gene expression by histone lactylation. Nature, 2019