
On July 27, 2024, the Xu Tao/Liu Huisheng research team of Guangzhou Laboratory/School of Biomedical Engineering of Guangzhou Medical University published an article titled "Directed differentiation of pancreatic delta cells from human pluripotent stem cells" online in the journal Nature communications.
This research article uses human pluripotent stem cells as the starting material to achieve a high degree of differentiation and maintenance of pancreatic lineage cells (especially endocrine precursor cells) through the combined application of fibroblast growth factors FGF7 and FGF2 from the posterior foregut stage of development to the pancreatic endodermal stage. This method promotes the efficient and directed differentiation of human pluripotent stem cells into delta cells by activating delta cell transcription factors. This process provides a potential source of delta cells for regenerative medicine and cell therapy, which can help research on the treatment of metabolic diseases such as diabetes.

Human islets are mainly composed of three endocrine cells: α, β, and δ, which secrete glucagon (GCG), insulin (INSULIN (INS), and somatostatin (SST) respectively. Previous research has focused heavily on the role of alpha and beta cells in regulating blood sugar homeostasis and the mechanisms that lead to diabetes. Recent evidence suggests that delta cells also play an important role in regulating blood sugar homeostasis. It inhibits the release of GCG and INS from α and β cells, and can participate in determining the blood sugar set point in vivo through the paracrine signaling pathway. Clinically, loss of delta cell function has also been found to cause islet dysfunction and blood sugar disorders. Therefore, delta cells have gradually become a potential target for diabetes treatment. However, delta cells only account for about 5% of human islets. Their isolation and purification are difficult, resulting in resource shortages and posing huge challenges to the study of the physiological functions of delta cells and drug development.
Functional islet α and β cells derived from human pluripotent stem cells have been shown to provide important cellular resources for basic research and clinical transformation research and accelerate the process of research and transformation. However, so far there is no method for directed differentiation of delta cells. Based on the similarity of the development trajectories of beta cells and delta cells, this study successfully established a method for efficient and directed differentiation from human pluripotent stem cells into functional islet delta cells, providing important resources for basic research on delta cells and screening of diabetes drugs.
This method uses human pluripotent stem cells Human Pluripotent stem cells (hPSCs) are based on the use of small molecules to induce endoderm (Definitive Endoderm, DE), original intestine (Primitive Gut Tube, PGT), posterior foregut stage (Posterior Foregut, PF), pancreatic endoderm Pancreatic Endoderm (PE), endocrine precursor cell (Endocrine Precursor (EP), Immature Endocrine Cell (IEC), Mature Endocrine Cell (MEC) and other stages, and finally functionally mature pancreatic delta cells are efficiently differentiated.
research process
The research team found that during the posterior foregut phase of differentiation into pancreatic endoderm, FGF7 concentration-dependently enhances the differentiation efficiency of pancreatic precursor cells; while extending the action time of FGF7 (pancreatic endoder-endocrine precursor cells) can further enhance the differentiation efficiency of endocrine precursor cells. Addition of FGF2, the specific activating ligand of FGFR1, at the same differentiation stage, can increase the differentiation efficiency of endocrine precursor cells and specifically promote the differentiation of delta cells while inhibiting the differentiation of α and β cells (the expression levels of HHEX and SST are increased, while the expression of GCG and INS are decreased). Finally, the combined use of FGF7 (50ng/ml) and FGF2 (20ng/ml) in the posterior foregut phase and pancreatic endoderm significantly promoted the directed differentiation of cells in the posterior foregut phase into delta cells, and its differentiation efficiency was about 4.3 times that of the group without FGF (the differentiation efficiency increased from 6.9% to 29.6%).
Immunofluorescence staining and flow sorting identified differentiated delta cells (SC-δ) as SST+ and HHEX+ and NKX6.1-. Morphologically, the dense vesicles enclosing SST were observed by transmission electron microscopy and gathered in the SC-δ cytoplasm in bands. These characteristics were consistent with the phenotype of adult pancreatic delta cells. Subsequently, samples differentiated into pancreatic endocrine precursor cells and mature SC-delta cells were subjected to Bulk RNA-seq sequencing, showing that the combination of FGF7 and FGF2 inhibited the development of liver and intestinal system cells (down-regulated TBX3 and NR5A2) and promoted the differentiation of pancreatic endocrine precursor cells and delta cells (up-regulated PDX1 and FGFR1). Single-cell sequencing analysis further revealed that stem cell-derived SC-delta cells have a similar transcriptome to adult islet delta cells and express related ion channel genes.
In vitro and in vivo experiments have further demonstrated that stem cell-derived delta cells are functional. Similar to human mature delta cells, SC-delta cells can promote SST secretion by SC-delta cells when stimulated by KCL(30mM), high glucose (20mM glucose), and K+ ion channel inhibitor Tolbutamide(10mM). At the same time, SC-delta cells were able to inhibit insulin secretion from β cells co-cultured with them (INS secretion in the presence of SSTR inhibitor CYN-154806(200nM) was 1.5 times higher than that in the group without inhibitor). Pancreatic delta cells secrete SST in a Ca2 + channel-dependent manner, which involves the influx of extracellular Ca2 + and intracellular Ca2 + oscillations in response to high glucose responses. Therefore, the dynamics of Ca2 + peaks reflect the functional maturity of delta cells.
The research team performed calcium imaging on differentiated SC-delta cells (SST-P2A-mCherry reporter system) and found that some cells showed dynamic calcium oscillations under high glucose (20G). To further study the functions of these cells in vivo, sorted SC-delta cells (3 million/mouse) were transplanted into the renal capsule of nude mice. Six weeks after transplantation, SST and CHGA immunostaining showed that most SC-delta cells were still alive. Glucose tolerance testing showed that mice transplanted with SC-δ cells had a certain degree of increase in glucose tolerance. At the same time, after 20 minutes of glucose load, the SST of mice transplanted with SC-δ cells increased significantly, while INS secretion decreased. These data suggest that differentiated SC-delta cells can inhibit the insulin secretion function of beta cells in vivo.
summary
This study provides a method for efficient and targeted induction and differentiation of pluripotent stem cells into delta cells in vitro. FGF2 and FGF7 jointly promote the differentiation of pancreatic endocrine cells and the specific expression of delta cell transcription activators, thereby promoting the differentiation of delta cells. This study also further revealed that human fibroblast growth factor may differentially regulate the development of pancreatic endocrine cells through different signaling pathways. This study not only provides important resources for basic research on delta cells and the development of diabetes treatment drugs, but will also promote clinical transformation research in diabetic regenerative medicine based on islet cell replacement therapy.
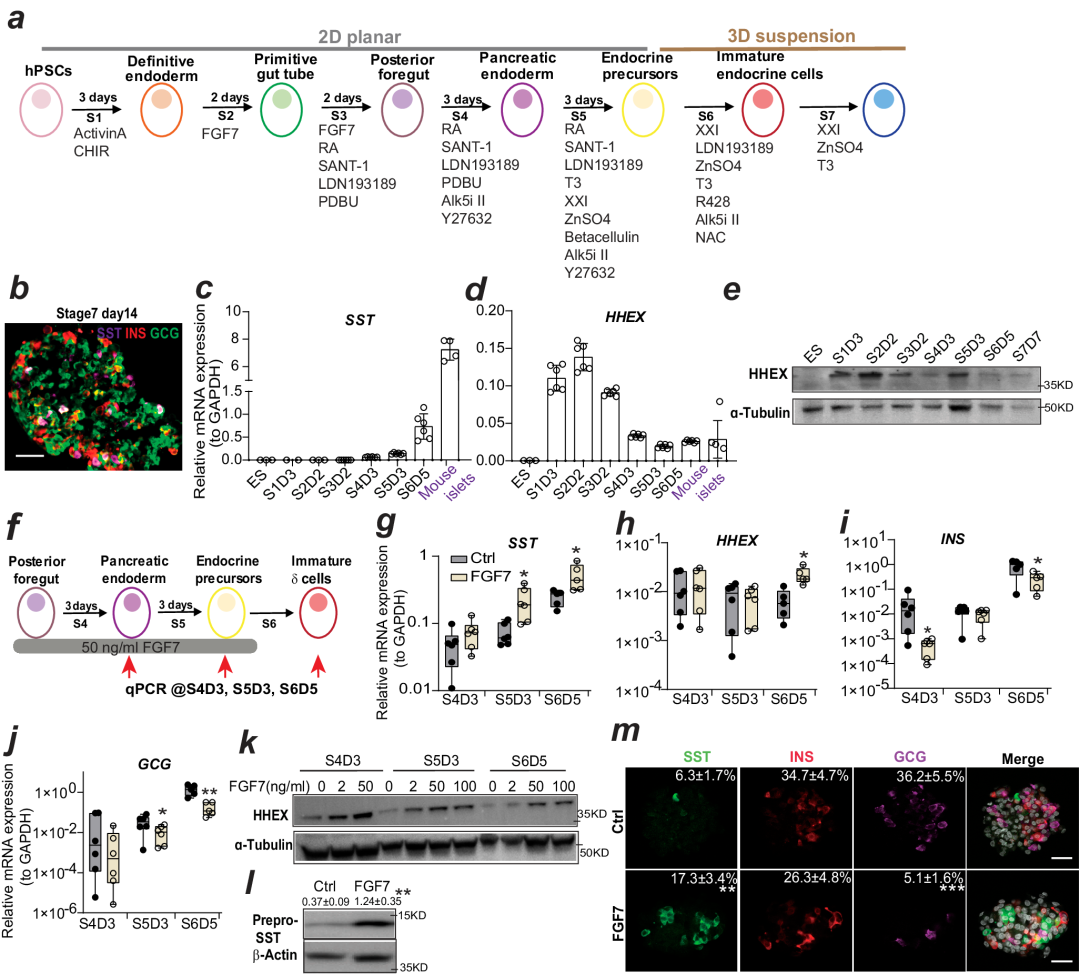
Prolonged FGF7 treatment induces CST and HHEX expression, thereby increasing delta cell production.
Original link: www.nature.com/articles/s41467-024-50611-7