
Military General Hospital: In the treatment of 65 patients with diabetes + fatty liver, it was shown that stem cells can effectively control blood sugar and improve liver function.
Mesenchymal stem cells have shown positive effects in improving blood sugar control, insulin sensitivity, fatty liver manifestations and lipid metabolism in patients with type 2 diabetes mellitus (T2DM) complicated with nonalcoholic fatty liver disease (NAFLD), and are expected to provide new treatment options for such patients.
Mesenchymal stem cells have shown positive effects in improving blood sugar control, insulin sensitivity, fatty liver manifestations and lipid metabolism in patients with type 2 diabetes mellitus (T2DM) complicated with nonalcoholic fatty liver disease (NAFLD), and are expected to provide new treatment options for such patients.
In June 2024, the General Hospital of the People's Liberation Army, in conjunction with the College of Medicine of the People's Liberation Army and the School of Medicine of Nankai University published an article in the Journal of the College of Medicine of the People's Liberation Army. Evaluation of the efficacy of umbilical cord mesenchymal stem cells (UC-MSCs) infusion in patients with type 2 diabetes complicated with nonalcoholic fatty liver disease: A clinical observation report of a post-hoc analysis of a prospective clinical trial.
This study elaborated on the effect of umbilical cord mesenchymal stem cell infusion in improving blood sugar control, IR, lipid metabolism, liver function and fatty liver in patients with T2DM complicated with NAFLD, and provided a new direction and idea for future treatment of this comorbidity.
Background: Type 2 diabetes mellitus (T2DM) and nonalcoholic fatty liver disease (NAFLD) are common complications. Co-infection of the two can aggravate complications and target organ damage. In recent years, mesenchymal stem cells are emerging in the treatment of metabolic diseases at home and abroad, but clinical research on them is relatively lacking.

Objective: To analyze the therapeutic effect of umbilical cord derived mesenchymal stem cell (UC-MSC) in patients with type 2 diabetes and nonalcoholic fatty liver disease.
65 cases of diabetes + fatty liver: Mesenchymal stem cells effectively improve blood sugar control and liver function
This study was based on a post-hoc analysis of a prospective, single-center, randomized, double-blind clinical trial (NCT02302599), which was approved by the Ethics Committee of the First Medical Center of the People's Liberation Army General Hospital.
Methods: The study prospectively recruited patients with diabetes and fatty liver who visited the First Medical Center of the General Hospital of the People's Liberation Army from October 2015 to December 2018, and selected patients according to inclusion and exclusion criteria. Patients who met the criteria were randomly divided into two groups, the umbilical cord mesenchymal stem cell (UC-MSC) group and the placebo group, which received UC-MSC intravenous infusion and placebo respectively.
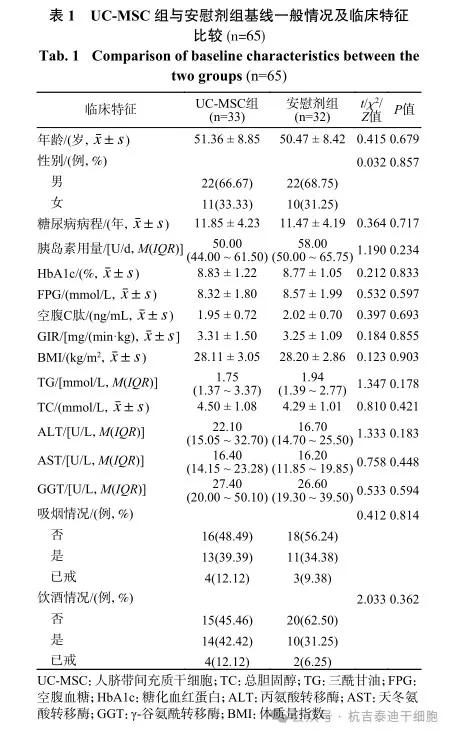
A total of 65 patients who met the criteria were included in this study, including 33 patients in the umbilical cord mesenchymal stem cell group and 32 patients in the placebo group. There were no statistical differences between the two groups in general baseline data such as age, gender, blood sugar control indicators, IR indicators and blood chemistry results. (See Table 1)
Stem cell injection method: Patients in the umbilical cord mesenchymal stem cell group were infused with 100mL of umbilical cord mesenchymal stem cell suspension into the median cubital vein, and 1×106/kg of umbilical cord mesenchymal stem cells were added according to the patient's body weight. Both groups received 3 infusions with an interval of 4 weeks.
Results
Comparison of lipid metabolism and liver function at different time points after umbilical cord mesenchymal stem cell therapy
TG levels usually refer to the amount of triglycerides in the blood.
TC usually refers to total cholesterol in medicine.
ALT (alanine aminotransferase) is an enzyme mainly found in liver cells. It is an important indicator for assessing liver health. It is often used to detect and monitor liver diseases, such as hepatitis, liver cirrhosis, fatty liver, etc.
GGT (γ-glutamyltransferase) is an enzyme that is mainly found in liver, pancreas, kidney, spleen, brain, lungs, bones and other tissues. Elevated levels of GGT often indicate possible damage or disease in the liver or bile duct.
TG levels in the umbilical cord mesenchymal stem cell (UC-MSC) group decreased significantly at weeks 9 and 20.
At week 20, TG levels in the umbilical cord mesenchymal stem cell group were lower than those in the placebo group [M(IQR): 1.34(1.15~1.97) mmol/L vs 1.89 (1.30~2.80) mmol/L, P=0.033]. (See Figure 1A)
TC was significantly lower than pretreatment levels at weeks 9 and 48 (P=0.002, P=0.038). (See Figure 1B).
ALT levels in the umbilical cord mesenchymal stem cell group decreased significantly at weeks 9 and 20 (P=0.036, P=0.001). (See Figure 1C)
GGT was significantly lower than baseline at weeks 9, 20, and 48 (P<0.005). (See Figure 1E)
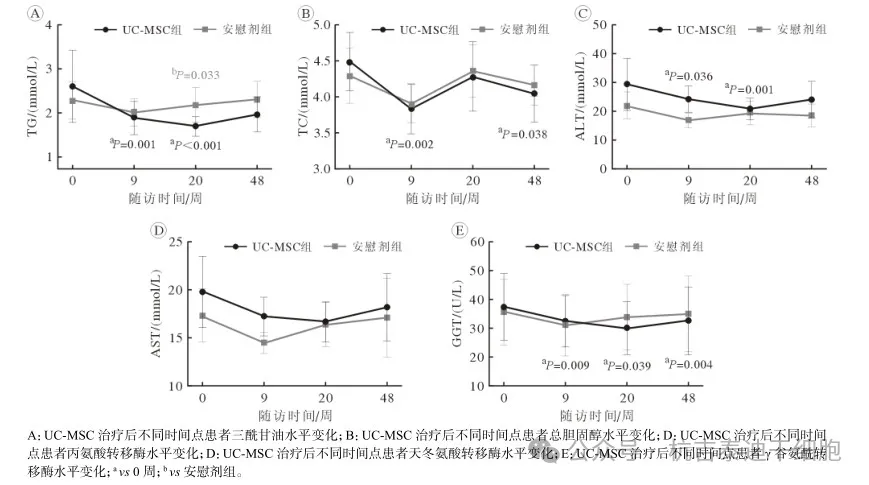
Figure 1: Changes in lipid metabolism and liver function indicators before and after treatment in the two groups
Comparison of reversal rates of fatty liver at different time points after umbilical cord mesenchymal stem cell therapy
At 20 weeks after treatment, ultrasound results showed that fatty liver was reversed in 15 patients in the umbilical cord mesenchymal stem cell (UC-MSC) group, which was significantly more than in the placebo group (45.45% vs 18.75%, P=0.021). The OR(95% CI) of the difference between the two groups was 3.611(1.177 - 11.083). (See Figure 2)
48 weeks after treatment, the number of cases of fatty liver reversal by liver ultrasound was 6 in both the umbilical cord mesenchymal stem cell group and the placebo group, with no statistical significance difference.
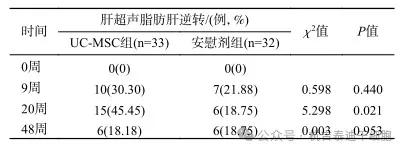
Figure 2: Comparison of liver ultrasound fatty liver reversal rates between the two groups at different follow-up time points
Comparison of changes in ZJU index and FIB-4 index at different time points after umbilical cord mesenchymal stem cell therapy
The ZJU Index is a biochemical scoring system used to assess the risk of nonalcoholic fatty liver disease (NAFLD).
The FIB-4 index (Fibrosis Index Based on Four Factors) is a blood biochemical indicator used to assess the degree of liver fibrosis.
The simple effects results showed that compared with baseline, the ZJU index in the umbilical cord mesenchymal stem cell group decreased significantly at weeks 9, 20 and 48 (P<0.05); at week 20, the ZJU index in the UC-MSC group was significantly lower than that in the placebo group (P=0.028). (See Figure 3A)
There was no significant change in FIB-4 index in both groups compared with baseline level at each time point (P>0.05).
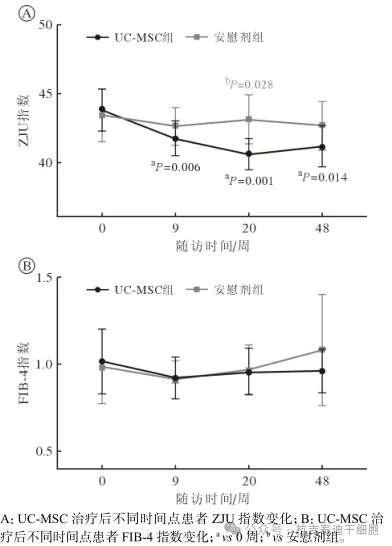
Figure 3: Changes in ZJU index and FIB-4 index in two groups before and after treatment
Comparison of blood sugar control indicators between two groups after umbilical cord mesenchymal stem cell therapy
FPG refers to fasting blood glucose.
HbA1c refers to glycosylated hemoglobin
In the umbilical cord mesenchymal stem cell treatment group, FPG at Week 9 was significantly lower than pretreatment levels (P=0.001) and was significantly better than the placebo group at Weeks 9 and 20 (see Figure 4A).
In addition, the HbA1c control target rates in the UC-MSC group at Weeks 9, 20 and 48 were significantly higher than those in the placebo group. (See Figure 4B)
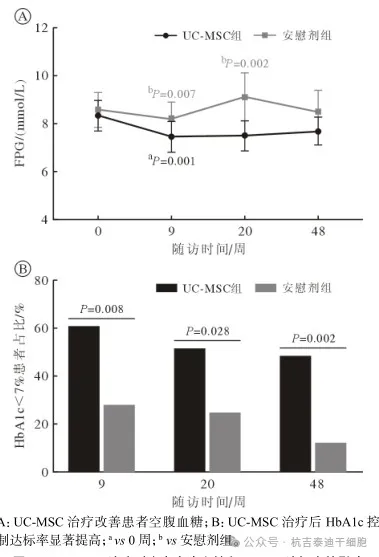
Figure 4: Effect of UC-MSC treatment on fasting blood glucose and HbA1c target rate
Comparison of IR improvement at different time points after umbilical cord mesenchymal stem cell therapy
GIR usually refers to the rate of glucose production.
GIR in the umbilical cord mesenchymal stem cell group was significantly improved at week 9 and week 48 after treatment, which were (4.05±1.69) mg/(min·kg) and (4.77±1.70) mg/(min·kg) respectively (P<0.001). There were no significant changes in the placebo group.
At week 48, GIR was significantly higher in the UC-MSC group than in the placebo group. (See Figure 5)
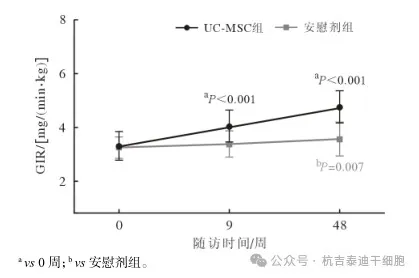
Figure 5: Line chart of changes in GIR values before and after treatment in two groups
Safety assessment
No major adverse events related to umbilical cord mesenchymal stem cell transplantation occurred in the patient.
Discussion: This post-hoc analysis selected cases of type 2 diabetes mellitus (T2DM) complicated with nonalcoholic fatty liver disease (NAFLD), and mainly discussed the therapeutic effect of umbilical cord mesenchymal stem cells on blood sugar control, IR, lipid metabolism, liver function, fatty liver and liver fibrosis improvement in patients with T2DM complicated with NAFLD.
The results showed that umbilical cord mesenchymal stem cell infusion significantly reduced the patient's fasting blood sugar. It is particularly worth mentioning that compared with the placebo group, more participants in the umbilical cord mesenchymal stem cell treatment group had HbA1c levels that reached the control target (<7.0%). This finding provides strong evidence for umbilical cord mesenchymal stem cells to improve blood sugar control in patients with T2DM and NAFLD.
In terms of liver ultrasound, the reversal rate of fatty liver in patients in the umbilical cord mesenchymal stem cell group after 20 weeks of treatment was as high as 45.45%, which was significantly better than that in the placebo group.
This study found that umbilical cord mesenchymal stem cell treatment significantly reduced the ZJU index and reached its lowest point at 20 weeks of treatment, which was significantly lower than the placebo group, further confirming the potential benefits of umbilical cord mesenchymal stem cells in improving NAFLD.
In addition, umbilical cord mesenchymal stem cell treatment also showed improved effects on lipid metabolism and liver function indicators such as TG.
As a core indicator in the pathological mechanism of NAFLD [2], the change trend of TG in this study was consistent with the improvement of NAFLD. It decreased from the baseline level at all time points during the follow-up period, and significantly improved compared with the placebo group at Week 20.
The lipid metabolism and liver function indicators such as TC, ALT and GGT in the umbilical cord mesenchymal stem cell group also showed varying degrees of improvement. It is worth noting that although the effectiveness of umbilical cord mesenchymal stem cell therapy has increased over time, indicators seem to tend to return to baseline levels at 48 weeks, suggesting that treatment may be time-sensitive.
Therefore, the results of this study support that the efficacy of umbilical cord mesenchymal stem cells improves over time after the first treatment, reaching the best effect at 20 weeks, but the long-term effectiveness and treatment continuity still need further research and exploration.
Conclusion
Studies have found that umbilical cord mesenchymal stem cell treatment reduces the daily insulin demand in patients with type 2 diabetes and fatty liver, reduces HbA1c levels, fatty liver manifestations and lipid metabolism, and improves insulin resistance in a time-dependent manner.
The patient did not experience major adverse events related to umbilical cord mesenchymal stem cell transplantation. Intravenous infusion of umbilical cord mesenchymal stem cells is a safe and effective method. Umbilical cord mesenchymal stem cell transplantation may be a potential treatment option for patients with type 2 diabetes and non-alcoholic fatty liver disease.