
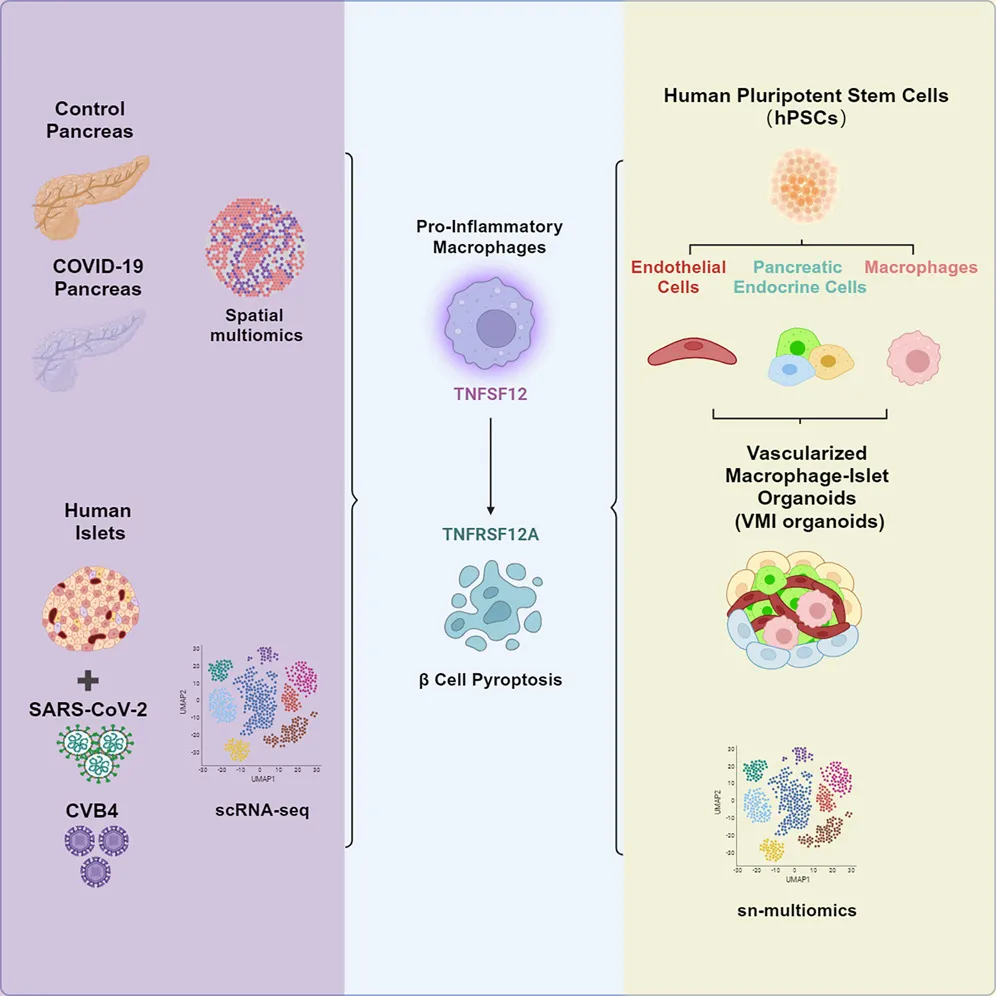
On September 3, 2024, Chen Shuibing and Robert E. The research team of Schwartz and Yang Liuliu (currently researchers at the Hematology Hospital/Institute of Hematology, China Academy of Medical Sciences) published a research paper titled "Human Vascularized Macrophage-Islet Organoids to Model Immune-Mediated Pancreatic β cell Pyrolysis upon Viral Infection" in the journal Cell.
The study found that in COVID-19 patients, pro-inflammatory macrophages induce pyrosis of islet beta cells through spatial multi-omic analysis of pancreatic tissue samples from control groups and COVID-19 patients and constructed A vascularized macrophage-islet organoids (VMI organoids) model derived from human pluripotent stem cells.

Pluripotent stem cells (PSCs) have the ability to self-renew and differentiate into multiple tridermal lineages. Cells and organoids derived from pluripotent stem cells can be applied to many aspects of basic research and clinical applications, including disease model construction, drug screening and cell therapy. Macrophages are polarized into different phenotypes under the stimulation of different environmental factors. For example, under the stimulation of LPS, INF-g, etc., macrophages show a pro-inflammatory phenotype and play a role in inflammation and tissue damage. Under the stimulation of IL-4, IL-13, etc., macrophages show an anti-inflammatory phenotype. Anti-inflammatory macrophages play an important role in wound, tissue damage repair, and angiogenesis.
In addition to these two phenotypes, there are multiple intermediate phenotypes in the polarization of macrophages. Pancreatic tissue contains many small units of islets. The islets include beta cells, alpha cells and delta cells, which are responsible for secreting insulin (INS), glucagon (GCG) and somatostatin (SST) respectively, thereby maintaining the stability of blood sugar. Damage to pancreatic beta cells is associated with the development of type 1 diabetes (T1D) and type 2 diabetes (T2D).
There is growing evidence that there is a strong link between the novel coronavirus disease (COVID-19) and diabetes. A study by the U.S. Centers for Disease Control and Prevention (CDC) reported that people under the age of 18 with COVID-19 are more likely to develop new diabetes than people who do not. In addition, after the beginning of the COVID-19 epidemic, the incidence of type 1 diabetes (T1D) and type 2 diabetes (T2D) increased significantly. In addition to the new coronavirus (SARS-CoV-2), many studies have shown a correlation between viral infection and T1D, including enteroviruses such as group B coxsackie virus, rotavirus, mumps virus and cytomegalovirus. Coxsackievirus B4(CVB4), a positive-sense single-stranded RNA virus isolated from newly diagnosed T1D patients, can infect in vitro and cause beta cell damage.

The team previously built a platform for human pluripotent stem cells to differentiate into multiple tissue cells and organoids of the tridermal layer. By infecting these differentiated cells and organoids with SARS-CoV-2, they first identified and reported that pancreatic endocrine cells can be infected by SARS-CoV-2. However, in addition to tissue and cell damage caused by direct virus infection, other factors, especially tissue and cell damage caused by inflammation, also play a key role in the disease progression of COVID-19 patients. However, there is currently a lack of suitable human disease models to study the tissue damage caused by immune cells during viral infection.
Research process
In order to test whether immune cells were involved in the damage of pancreatic islet beta cells, the team collected pancreatic tissue samples from the control group and COVID-19 patients and conducted spatial multi-omic analysis. The study found that compared with the control group, the proportion of insulin (INS)-expressing beta cells in the islets of COVID-19 patients was significantly reduced. In addition, pro-inflammatory macrophages are activated and enriched in the islets of COVID-19 patients. Afterwards, the team isolated human islet tissue cells and conducted them for infection with SARS-CoV-2 and CVB4 and single cell transcriptome analysis (scRNA-seq). Studies have found that both SARS-CoV-2 and CVB4 can lead to the activation of pro-inflammatory macrophages in the islets and the pyrodeath of pancreatic beta cells.
In order to investigate whether the pyroapoptosis of COVID-19 patients and virus-infected pancreatic beta cells was caused by direct viral infection or induced by the activation of pro-inflammatory macrophages, the team constructed a vascularized macrophage derived from human pluripotent stem cells. -islet organoid model. The team first differentiated embryonic stem cells (hESCs) into pancreatic endocrine cells (including beta cells, alpha cells and delta cells), macrophages and endothelial cells through various factors, and then the differentiated cells were optimized. Assemble into VMI organoids under organoid culture conditions.
The team further conducted immunofluorescence staining analysis and functional evaluation of key cellular components in this type of organ model, and found that the presence of macrophages and endothelial cells promoted the function of beta cells in responding to high concentrations of glucose and KCL. At the same time, endothelial cells and macrophages also retain their unique functions in this type of organ model. By further inducing and constructing VMI organoids containing pro-inflammatory macrophages in vitro, the team also demonstrated that pro-inflammatory macrophages can indeed cause pyroapoptosis of pancreatic beta cells.
graphical summary
In terms of molecular mechanisms, the research team analyzed and verified the cell chat between macrophages and pancreatic beta cells, and found that pro-inflammatory macrophages induce pancreatic beta cells through the TNFSF12-TNFRSF12A signaling pathway. The use of neutralizing antibodies to TNFSF12 can alleviate viral (SARS-CoV-2 or CVB4) induced apoptosis of beta cells in islets or pro-inflammatory macrophage induced organoid models. Finally, the researchers also confirmed pyroapoptosis of pancreatic beta cells in the pancreas in pancreatic tissue samples from patients with COVID-19, and this phenomenon was not related to diabetes.
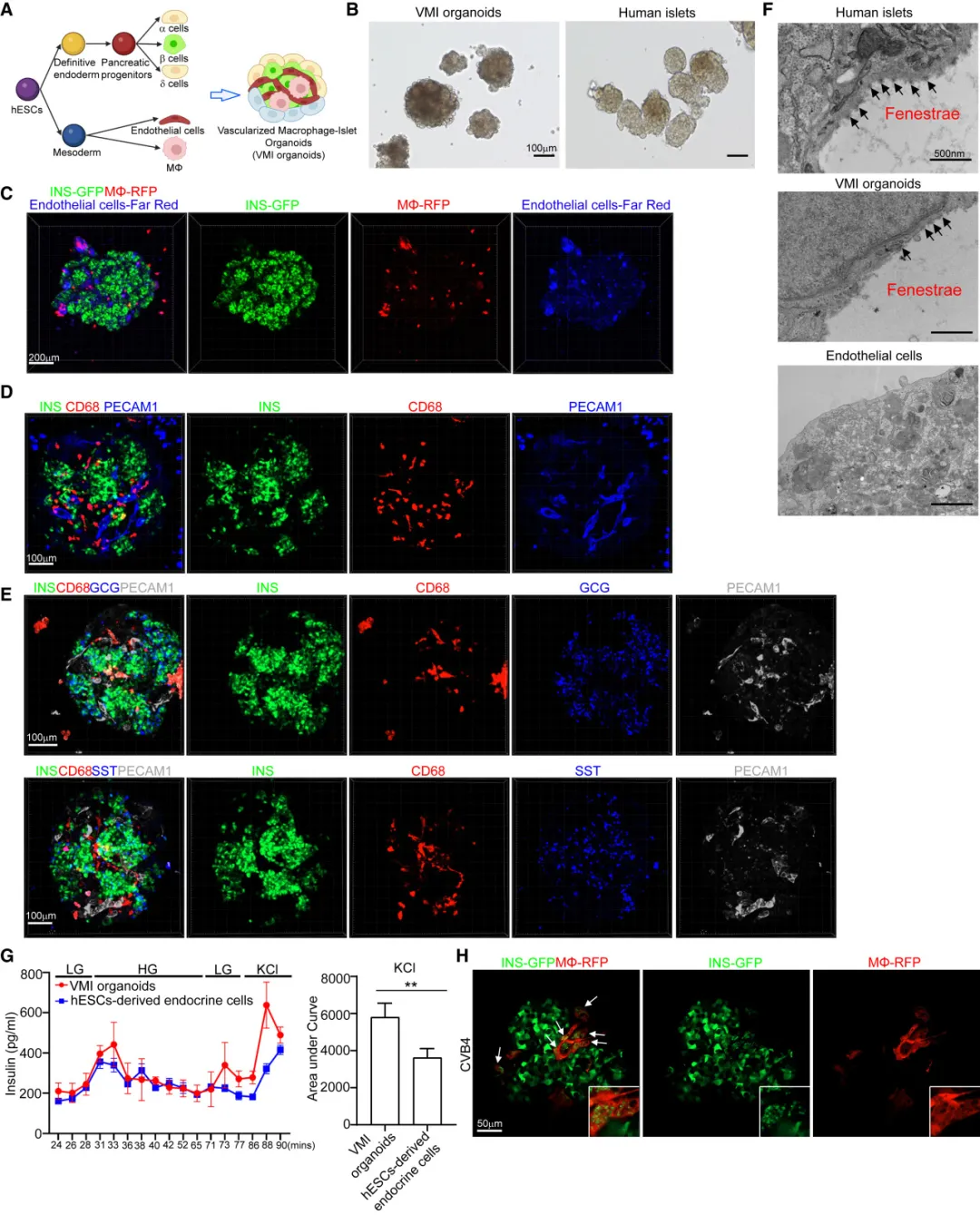
Construction of hPSC-derived VMI organoids
Summary
Immune cells play an important role in tissue homeostasis and the occurrence and development of diseases, especially infectious diseases. However, few human models have studied immune cell-mediated damage to host tissues and tissues. This study constructed human pluripotent stem cell (hPSC)-derived vascularized macrophage-islet organoids (VMI organisms), providing a valuable tool for studying immune cell-mediated damage to host tissues and cells, and revealing the potential damage mechanism of beta cells during virus exposure.
Original link: doi.org/10.1016/j.stem.2024.08.007