
In recent years, stem cell therapy has become a research hotspot in the biomedical field, and donor stem cells have been used for decades in multiple research and clinical treatments. Current cell transplants include multiple types of stem cells, such as ectomenchymal stem cells, hematopoietic stem cells, and stem cell derivatives such as vesicles and extracellular vesicles. With the advancement of cell engineering technology, the application of stem cell therapy has entered a new stage, significantly expanding its therapeutic potential.

Induced pluripotent stem cells (iPSCs) are an important breakthrough in the field of modern biomedicine, opening up new horizons in disciplines such as biology, pathophysiology and cell regenerative medicine. Cells derived from iPSCs are now an emerging direction in cell therapy research, and induced mesenchymal stem cells (iMSCs) differentiated from iPSCs represent the forefront of mesenchymal stem cell (MSCs) research.
Experimental evidence shows that iMSCs have greater proliferation capabilities than natural MSCs and reduce age-related changes and heterogeneity. A large number of clinical trials have further shown that iMSCs may have better application prospects in cell therapy. This article summarizes the basic research and clinical trials of iMSCs, aiming to provide reference and reference for future research.
Introduction
Mesenchymal stem cells (MSCs) have the ability to differentiate into multiple mesodermal cell types, such as fat cells, chondrocytes, bone cells, and muscle cells. Their definition relies on the expression of specific surface markers such as CD73, CD90 and CD105, and do not express CD45, CD34, CD14 or CD11b, CD79 or CD19 and HLA-DR. With the advancement of research, scientists have gradually increased their controversy over the definition of MSCs, especially as the understanding of the heterogeneity of MSCs from different tissue sources deepens.
Studies have identified MSCs in many tissues, such as umbilical cord, bone marrow, adipose tissue, synovial membrane and pulp. MSCs from different sources have differences in surface markers, and their differentiation potential also has subtle differences, which is reflected in their different abilities to differentiate into fat cells, chondrocytes and bone cells. Although these characteristics have been extensively discussed in many literature, this article will not repeat them. The importance of studying the heterogeneity of MSCs is that they are the main source of current cell therapies. Although they have shown positive therapeutic effects in many preclinical studies and trials, their heterogeneity is also closely related to the variability of clinical trial results.
Advances in technologies such as single-cell RNA sequencing (scRNA-seq) have allowed researchers to deeply analyze differences in MSCs from different individuals and tissues. For example, Wang et al. analyzed seven tissue-specific subgroups with different gene expression profiles and identified five conserved MSCs subtypes. Umbilical cord derived MSCs (UC-MSCs) have shown advantages in immunosuppressive ability. Xie et al. identified some subsets of bone marrow MSCs through scRNA-seq, including CD26 + osteogenic subtype, CMKLR1 + functional subtype, and proliferative subtype. Zhang et al. studied freshly isolated, uncultured umbilical cord derived MSCs using scRNA-seq technology. The results showed that after cultured in vitro, MSCs differentiated into subsets with immunoregulation, osteogenesis and chondrogenesis potential.
Breakthroughs in iPSCs and their application in MSCs research
Induced pluripotent stem cells (iPSCs) are produced by reprogramming differentiated somatic cells, usually by introducing specific transcription factors. This process allows differentiated cells to restore pluripotency and allow the formation of cell lines similar to embryonic stem cells. At present, the technology for producing iPSCs is highly mature, and major breakthroughs have been made in the optimization of induction and differentiation methods. This technological advancement has led to remarkable results in differentiation research of iPSCs, including differentiation into mesenchymal stem cells (MSCs).
Compared with tissue-derived MSCs, iMSCs show many advantages. First, tissue-derived MSCs have more obvious replicative aging, while iMSCs have less heterogeneity. Second, iMSCs can be generated from donor or patient cells and become an important source of personalized cell therapy. In addition, regardless of donor age or cell origin, iMSCs show genetic characteristics of rejuvenation. Therefore, as a key cell source for future cell therapies, iMSCs occupy an important position in modern research.
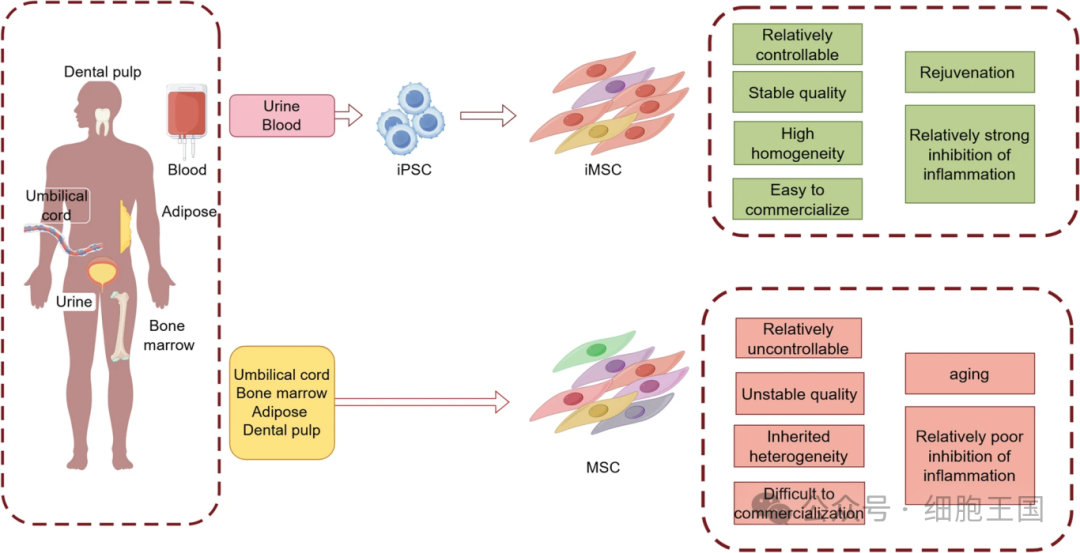
Comparison of iMSC and tissue derived MSC
Induction method of iMSCs
In the past decade, significant progress has been made in methods to induce differentiation of iPSCs into MSCs. Various existing technologies can promote the differentiation of iPSCs into iMSCs, but the iMSCs produced under different methods may be different, and these differences are crucial in their clinical application. For example, Lian et al. successfully induced the differentiation of MSCs by using specially designed induction medium instead of the medium for iPSCs. The medium consisted of standard DMEM and FBS, supplemented with 10 ng/mL basic fibroblast growth factor (bFGF), platelet-derived growth factor AB (PDGF-AB) and epidermal growth factor (EGF). After 10 days of induction, iMSCs were successfully generated. Tran et al. added 5 ng/mL Activin A, 2 μM BIO and 20 ng/mL BMP to basic medium for induction for 3 days, and then continued culture with 10 ng/mL bFGF and EGF for 10 days to complete the generation of iMSCs.
These induction methods are basically consistent in terms of the criteria for identifying MSCs, and emphasize the important role of bFGF in the induction process. bFGF is a polypeptide that promotes mesodermal cell division and angiogenesis, and can significantly improve the efficiency of iMSCs generation. Whether there are phenotypic differences in iMSCs generated by different induction methods remains to be further studied. During the induction process, cost and efficiency issues need to be comprehensively considered. At the same time, commercially available finished kits also provide cost-effective options.
Comparison of iMSCs and tissue-derived MSCs
In the induction research of iMSCs, scientists have been thinking about a question: Do iMSCs have specific advantages compared with MSCs extracted directly from organisms? The results show that there are many differences between iMSCs and MSCs in proliferation ability, gene expression and function. Researchers have generally found that iMSCs have strong adipose differentiation ability, while tissue-derived MSCs have outstanding immunoregulatory functions. In terms of immune-related functions, iMSCs show stronger immunosuppressive ability. Compared with UC-MSCs, iMSCs have higher expression of anti-inflammatory factors, which makes them more potential in the treatment of immune-related diseases.
The potential of iMSCs in cell therapy
iMSCs have shown great potential in the treatment of multiple diseases, especially in conditions related to ischemia and inflammation, such as myocardial infarction, lower limb ischemia, inflammatory bowel disease (IBD), and acute lung injury. In the study of these disease models, the main function of iMSCs is to regulate immune responses and promote tissue repair, similar to the role played by traditional MSCs in vivo. However, the specific functions of iMSCs may vary depending on different pathological environments. For example, Hynes et al. found that iMSCs significantly promoted the formation of newly mineralized tissue in a rat periodontitis model and contributed to the regeneration of periodontal tissue. Similarly, Lian et al. found that iMSCs have good repair effects in limb ischemia in mice. In a mouse model of IBD, iMSCs promoted the healing of intestinal mucosa by secreting TSG-6, significantly improving the regenerative ability of intestinal tissue in mice.
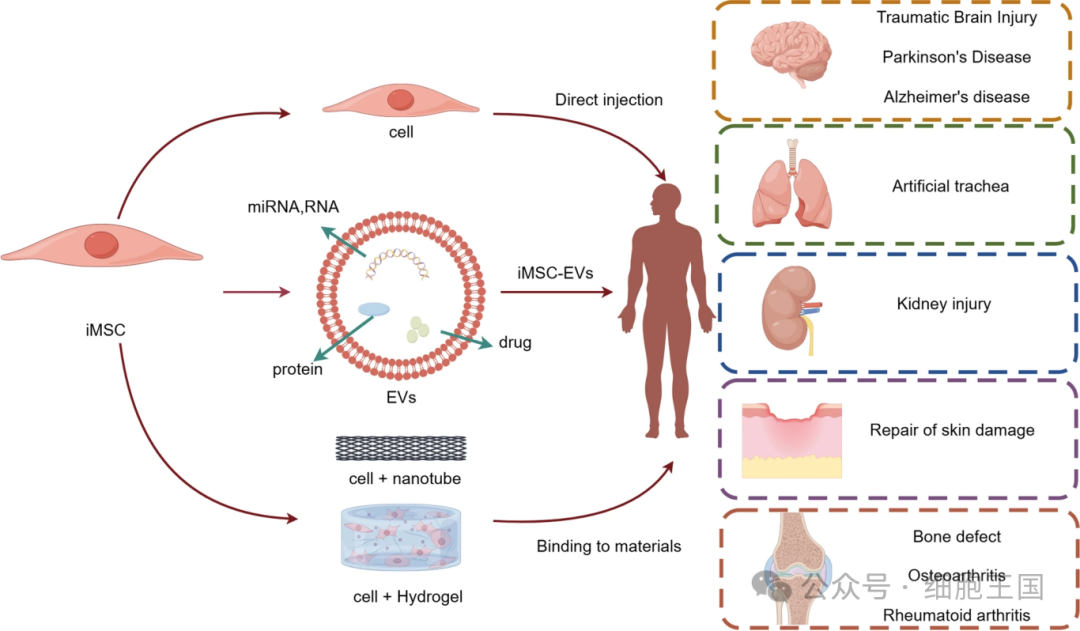
Application of iMSCs in cell therapy research
In addition, iMSCs have also shown potential in protecting retinal ganglion cells (RGCs). Studies have found that iMSCs can transfer through mitochondria, helping maintain the survival of RGC and restoring their retinal function in mice with retinopathy. In asthma inflammation models, iMSCs significantly improve epithelial cell dysfunction by forming junction channels (TNTs) with epithelial cells to transfer healthy mitochondria into damaged cells. These studies suggest that iMSCs restore tissue function mainly through paracrine signaling or mitochondrial transfer, and that they have a relatively low probability of self-differentiation in vivo.
Application of iMSC-derived extracellular vesicles (EVs) in cell therapy
Extracellular vesicles (EVs) are small vesicles secreted by cells. They contain cellular components such as DNA, RNA, lipids and proteins. They can transfer information between cells and affect the behavior of recipient cells. Because EVs have molecular transport capabilities and can reduce immune responses more effectively than direct transplantation of cells, EVs have gradually become a key tool in cell therapy.
A large number of studies have confirmed that EVs from MSCs have potential in the treatment of multiple diseases, and current research is focusing on how to optimize and modify these EVs to improve their therapeutic effectiveness. Similarly, iMSCs are also capable of secreting EVs, and because their proliferation ability is stronger than traditional MSCs, they show significant advantages in external secretion and therapeutic applications.
In the study of bone and joint diseases, Zhu et al. compared the EVs secretion characteristics of iMSCs and synovial-derived MSCs (SM-MSCs) in an osteoarthritis (OA) model. Studies have shown that although there are no significant differences in particle size and surface markers between the two EVs, iMSC-derived EVs have better efficacy in promoting chondrocyte migration. In addition, Cui et al. developed an exosome platform with bone-targeting capabilities that can deliver siRNA to osteoblasts, thereby enhancing osteoblast differentiation and inhibiting osteoclast production, which provides new possibilities for the treatment of osteoporosis.
Studies have also shown that iMSC-EVs are more effective in delaying the progression of disc degeneration than traditional EVs. iMSC-EVs demonstrate anti-aging properties by transporting miR-105- 5p to aging nucleus pulposus cells and activating the Sirt6 pathway. In stroke models, iMSC-EVs can reduce infarction volume, promote angiogenesis, and reduce neurological damage.
In tumor treatment, iMSC-EVs have also shown strong potential, especially in the treatment of triple negative breast cancer (TNBC). Studies have shown that iMSC-EVs show stronger cytotoxic effects in adriamycin resistant TNBC cells, superior to traditional free or liposomal forms of doxorubicin. In addition, iMSC-EVs can also enhance the anti-tumor immune response by activating the STING pathway and inducing IFNβ expression in monocytes. Studies have shown that early-passed iMSC-EVs have higher immunomodulatory efficacy than late-passed EVs. Due to the low heterogeneity and strong expansion ability of iMSCs, their secretions have greater development potential in clinical applications.
Therapeutic strategies for combining iMSCs with biomaterials
Existing studies have shown that iMSCs combined with a variety of biomaterials can produce significant tissue repair effects. For example, using iMSC-EVs in conjunction with hydrogels can significantly reduce scar formation and accelerate wound healing. Using 3D printed hydrogel scaffolds in conjunction with iMSCs can optimize their survival environment after transplantation and improve their survival rate in vivo. The application of this method in endometrial injury models shows that iMSCs can completely restore the structure and function of the endometrium, and partially restore the ability to implant embryos and maintain pregnancy.
By combining with scaffold materials, the application potential of iMSCs in tissue regeneration and repair has been further enhanced. For example, Kim et al. developed a double-layered tubular stent to promote the regeneration of tracheal mucosa and cartilage. The scaffold framework consists of electrospun polycaprolactone (PCL) nanofibers and 3D printed PCL microfibers. The cells implanted in the scaffold include human bronchial epithelial cells (hBECs), iMSCs and iPSC-derived chondrocytes, which have been successfully promoted in vivo. Regeneration of the trachea. The strategy of combining this material with iMSCs shows great application potential in biomimetic tissue and organ regeneration and transplantation.
Challenges facing iMSCs
The advantages of iMSCs include rich sources, sufficient number, good therapeutic effects, high homogeneity, and can effectively solve immune rejection and ethical issues. As derivatives of iPSCs, iMSC-EVs may be comparable to or even surpass MSCs-EVs in some therapeutic effects. However, there are still some key issues to be solved during the clinical transformation of iMSCs. First, the characteristics of the reprogrammed iPSCs require further study. Although their stem nature is similar to embryonic stem cells, the specific differences are not clear.
Secondly, the potential tumorigenic risks brought by iPSCs reprogramming technology cannot be ignored. With the development of small molecule reprogramming technology for iPSCs, the quality control of iPSCs and potential MSC sources have been further guaranteed. In theory, iPSCs have the ability to differentiate and self-renew from multiple lines, giving them great potential for large-scale production and addressing tissue heterogeneity. However, whether iMSCs can retain their characteristics under stress conditions (such as inflammation or hypoxia) still needs to be further verified through experiments.