
In the current era of rapid advancements in medical technology, pluripotent stem cell therapy, with its tremendous potential and continuous breakthroughs, has become the core focus of the global medical field.
Recently, this cutting-edge field has witnessed a series of exciting advancements - not only have they opened up brand-new treatment paths for many intractable diseases, but they have also injected strong impetus into the development of the entire regenerative medicine, making the hope of conquering difficult and complicated diseases increasingly clear.
01 HaloTag-PIEZO1
PIEZO1 is a mechanically gated ion channel that plays a core role in sensing physiological mechanical stimuli such as blood flow, tissue tension, and cell traction. However, researchers have long lacked a non-invasive tool that can both locate the endogenous PIEZO1 and monitor its channel activity in real time.
Recently, a team from the University of California in the United States has developed a novel HaloTag-PIEZO1 human induced pluripotent stem cell (hiPSC) platform, successfully "dynamically visualizing" the spatial localization and functional state of PIEZO1 in multiple differentiated cell types and organoids, providing a powerful tool for studying the mechanism of human force-sensing channels and disease modeling.
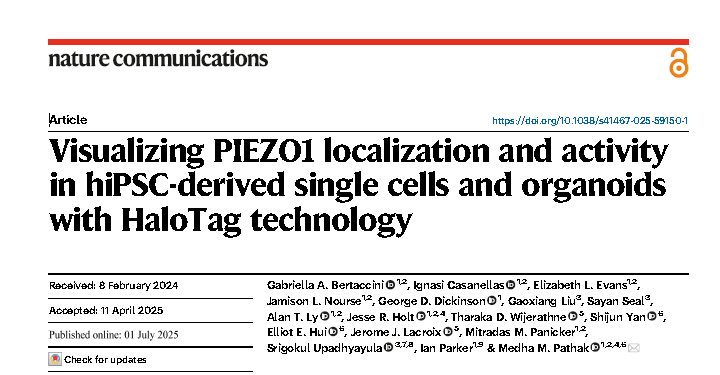
The core value lies in:
1. Endogenous & humanized: Directly labeling endogenous PIEZO1 at the human genome level avoids overexpression interference, and the results are more physiologically relevant.
2. Versatility: HaloTag is compatible with multiple ligands. This study focuses on imaging (localization and activity), but its potential goes far beyond this (such as protein interaction research, efficient purification, and targeted degradation of Protacs).
3. High-performance imaging: The ultra-high brightness and strong anti-photobleaching property of JFHTLs enable the accuracy, duration and signal-to-noise ratio of localization imaging to far exceed those of traditional fluorescent proteins (such as tdTomato), and for the first time clearly reveal the heterogeneous diffusion pattern of PIEZO1 and its dynamic enrichment in migrating cells.
4. Revolution in single-channel activity monitoring: The Ca²⁺ -sensitive JF646-BAPTAHTL, combined with the positioning advantage of HaloTag, has for the first time achieved non-invasive optical recording of the activity of endogenous PIEZO1 in a single channel with a time resolution close to that of a patch clamp (~4ms) in intact cells and tissues, and can simultaneously track the activity of mobile channels.
5. Multi-scale capability: The platform can differentiate into various cell types (ECs,NSCs, keratinocytes) and complex three-dimensional organoids (MNRs). In particular, by using a light sheet microscope, the molecular-scale localization of PIEZO1 in living human organoids and the visualization of its activity in a three-dimensional tissue environment were successfully achieved, revealing the tissue mechanics basis of its spatial distribution and activation.
6. Revealing new Biology: Discovering the inherent differences in PIEZO1 activity among different human cell types (NSCsvs, ECs), as well as the spatially specific regulatory patterns in neural organoids where PIEZO1 is enriched in the lumen but has a higher activation efficiency at the outer edge.
PIEZO1-HaloTag hiPSC系的生成和验证
This research employs ingenious genetic engineering (endogenous PIEZO1-HaloTag hiPSC), innovative chemical probes (high-performance JF HTL, especially the Ca²⁺ -sensitive JF646-BAPTA), and cutting-edge imaging techniques (TIRF, super-resolution tracking, particularly the light plate microscope AO-LLSM). A powerful integrated platform has been established. It not only overcomes many limitations of existing technologies, but also for the first time achieves high-precision localization, dynamic tracking and ultra-high temporal resolution single-channel activity monitoring of endogenous PIEZO1 in human physiological-related model systems (from single cells to three-dimensional organoids). This work represents a significant breakthrough in the field of mechanosensitive ion channel research, which will greatly enhance our understanding of the complex role of PIEZO1 in human health and disease, and lay a solid foundation for the development of future therapeutic intervention strategies.
02 High-throughput robot separation technology
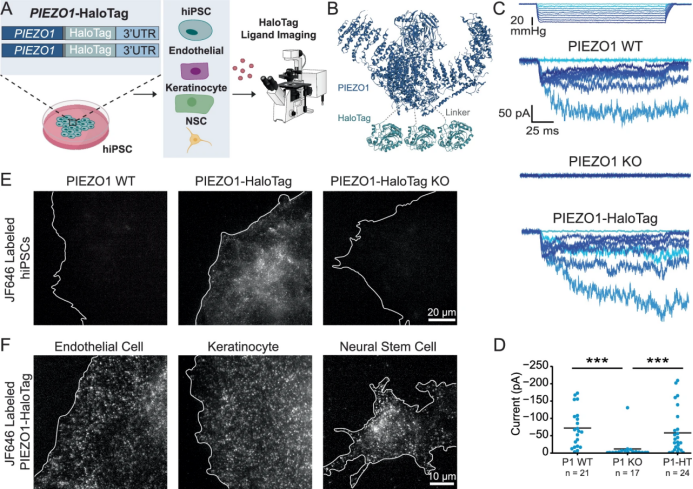
Recently, the Takahashi team from the Tokyo Institute of Medical Sciences in Japan published a breakthrough study in the journal Stem Cell Research & Therapy. They developed a high-throughput robotic separation technology, which for the first time systematically revealed the high-frequency phenomenon of CRISPR-Cas9 inducing homozygous gene mutations in human iPS cells, laying an important foundation for the precise application of gene editing in regenerative medicine.
The research team designed a three-dimensional culture system, encapsulating individual iPS cells in a "dome" formed by Matrigel (matrix gel), providing the cells with a supporting structure similar to the in vivo microenvironment. Meanwhile, the CELL HANDLER robot system is introduced. By using its visual recognition system, cell clusters from monoclonal sources can be precisely screened through parameters such as diameter and roundness, and cell clusters with diameters of only 100-200 micrometers can be identified and separated.
Through in-depth analysis of over 1,000 gene-edited iPS cell clones, the team found that the frequency of homozygous editing mutations was much higher than that of heterozygous mutations. For the first time, they achieved systematic profiling at the single-cell level, providing key data for understanding the randomness and regularity of gene editing.
Robotic isolation of human iPS cell clones for genome editing
This research achievement has laid an important foundation for the precise application of gene editing in regenerative medicine, which is conducive to promoting the development of gene editing technology in disease treatment and other aspects, and has taken an important step towards achieving goals such as personalized cell therapy.
03 iPSC- Organoids
Recently, a research team from the University of California, Los Angeles (UCLA) successfully cultivated lung and intestinal organoids with complete vascular networks using induced pluripotent stem cells (iPSCs). These micro-organs not only have a highly similar structure to real organs, but also possess corresponding physiological functions, providing powerful new tools for disease research, drug testing and personalized treatment.
In this study, the research team precisely regulated the bone morphogenetic protein (BMP) signaling pathway to simultaneously differentiate mesoderm and endoderm cells in three-dimensional embryonic bodies, achieving vascularization of lung and intestinal organoids. This innovative approach not only optimizes the cell type diversity, three-dimensional structure and maturity of organoids, but also significantly improves the cell survival rate. In the experiment, they found that by adjusting the timing and intensity of the BMP signal, the proportion of mesoderm and endoderm could be balanced in the early stage, thereby influencing the formation of the anterior-posterior axis (A-P) pattern of organoids and laying the foundation for subsequent tissue specialization.
The research team applied this technology to a rare congenital disease called alveolar capillary dysplasia with pulmonary vein dislocation (ACDMPV). This disease is caused by mutations in the FOXF1 gene, leading to abnormal development of pulmonary blood vessels. Currently, there is no effective treatment for it.
By cultivating vascularized lung organoids from patient stem cells, researchers have for the first time successfully reproduced the vascular defects and secondary epithelial abnormalities of the disease. This breakthrough achievement not only validates the potential of the new model in disease research, but also provides a new perspective for revealing the role of intercellular interactions in disease occurrence.
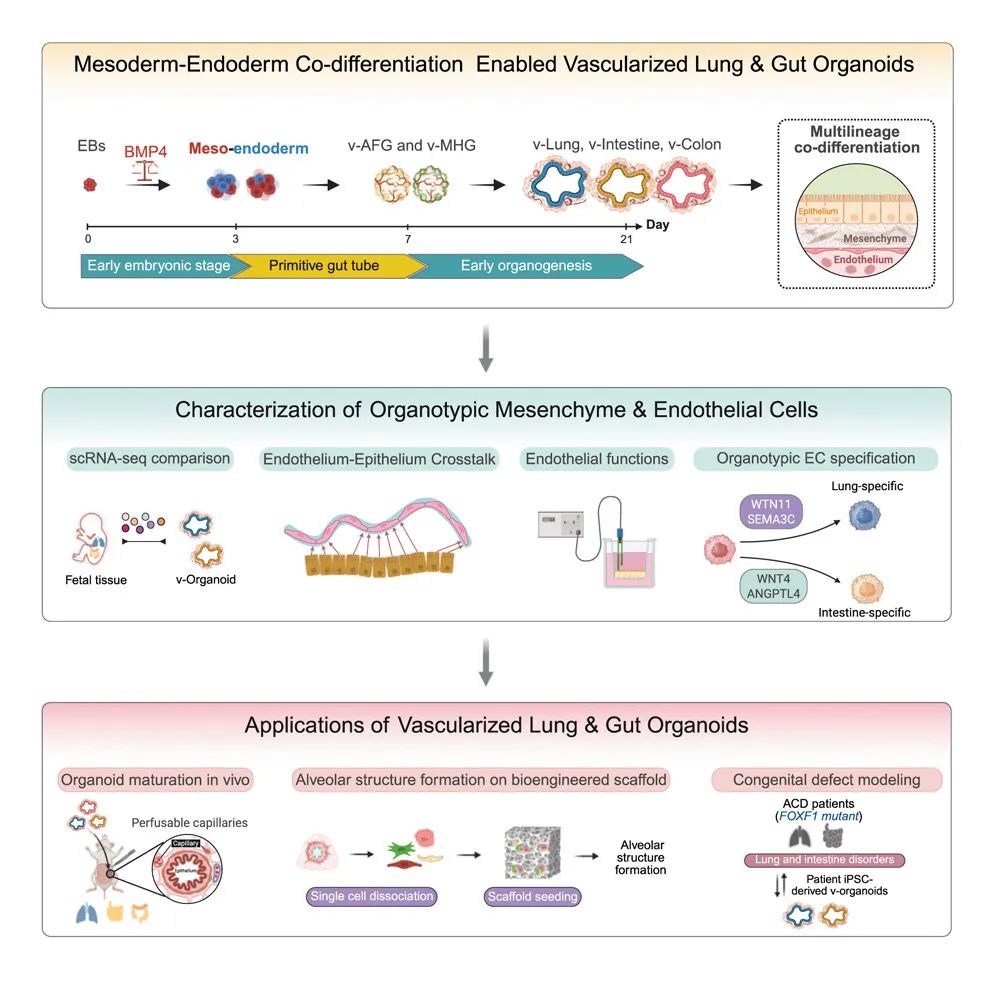
Graphic summary
This research not only provides a new perspective for the mechanism study of lung and intestinal diseases, but also opens up new paths for personalized medicine and regenerative medicine. In the future, these vascularized organoids are expected to be combined with microfluidic chip technology to study the dynamic interaction between immune cells and endothelial cells, and even explore the mechanism of transplant rejection.
The research team emphasized that although the current organoid models are not yet fully mature, by further optimizing the culture conditions, such as introducing oxygen gradients and mechanical stimulation, it is expected to achieve functional characteristics closer to those of adult organs. The wide application of this technology will inject new vitality into the research of organ-on-a-chip and human model systems.
The above three studies have promoted the development of pluripotent stem cell therapy and related fields from different dimensions, jointly demonstrating the huge potential of pluripotent stem cell technology in exploring life mechanisms, conquering difficult diseases and advancing regenerative medicine, and also opening up broader space for the innovative development of the medical field in the future.