
In the current era of rapid advancements in medical technology, pluripotent stem cell therapy, with its tremendous potential and continuous breakthroughs, has become the core focus of the global medical field.
Recently, this cutting-edge field has witnessed a series of exciting advancements - not only have they opened up brand-new treatment paths for many intractable diseases, but they have also injected strong impetus into the development of the entire regenerative medicine, making the hope of conquering difficult and complicated diseases increasingly clear.
01 iPSC-CAR-NK
On June 24th, Professor Xu Huji's team from the Second Affiliated Hospital of Naval Medical University (Shanghai Changzheng Hospital) published a breakthrough in the journal Cell.
The team was the first to apply the dual-target CAR-NK cell therapy (QN-139b) derived from iPSC (induced pluripotent stem cells) in clinical practice, successfully reversing the condition of a patient with refractory systemic sclerosis.
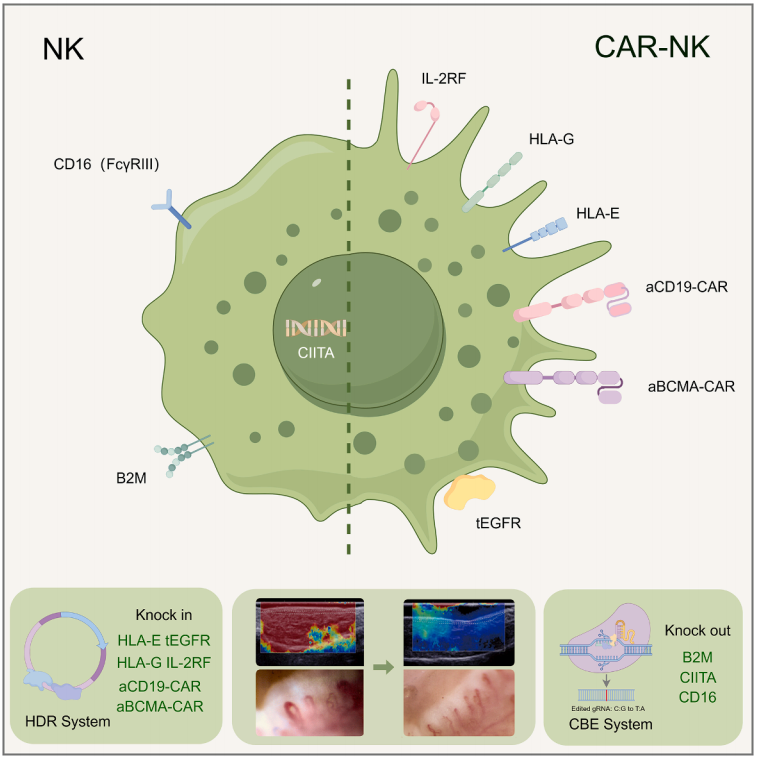
Schematic diagram of the QN-139b research process
In a clinical trial, a 36-year-old patient with a history of refractory systemic sclerosis for nearly 20 years received QN-139b treatment for the first time on August 4, 2024. The treatment dose was 6×10⁸ cells, administered in four doses (days 0, 3, 7, and 10). After a 6-month follow-up, QN-139b demonstrated good overall safety, and no special discomfort was reported by the patients. Clinical assessment shows that the patient's subjective symptoms have significantly improved, and objective indicators have significantly improved: the level of autoantibodies has significantly decreased, complement has returned to normal, the modified Rodnan skin score (mRSS) has significantly decreased, and the combined response index (ACR-CRISS) of the American College of Rheumatology and Lupus Study Group has significantly improved.
This research is the world's first successful case of IPSC-derived CAR-NK cells treating autoimmune diseases, verifying the treatment concept from "targeted clearance" to "regenerative repair", and establishing a new paradigm for the precise treatment of autoimmune diseases.
02 iPSC- Organoids
On June 30th, the research team led by Gu Mingxia from Cincinnati Children's Hospital/University of California, Los Angeles, in collaboration with the team led by Guo Minzhe from Cincinnati Children's Hospital, published a research paper in the top international academic journal Cell. This study has for the first time achieved a tissue-specific vascular bed derived from human IPscs: the simultaneous construction of functional vascular networks in lung and intestinal organoids.
The research team established an in vitro vascularized organoid platform based on developmental principles. This platform truly reproduces the coordinated development of mesoderm and endoderm lineages. This method can achieve efficient differentiation and lineage specialization of endoderm derivatives and organotype endothelial/mesenchymal cell populations.
The traditional construction method requires the addition of more than ten factors, while this method only needs one inhibitor, Noggin, to cultivate lung organoids and three activators (CHIR99021, FGF4, VEGFA) to cultivate intestinal organoids. Cells in 3D culture spheres will spontaneously secrete vascular growth signal molecules, naturally forming a vascular network. The resulting vascularized lung and intestinal organoids have organ-specific endothelium and mesenchyme. The diversity of cell types, three-dimensional structure, survival rate and maturity have all been enhanced, and they possess real physiological functions - lung vessels form a tight barrier (simulating gas exchange), and intestinal vessels are highly permeable (facilitating nutrient absorption). After being transplanted into mice, the organoid blood vessels could bind to the host circulatory system, maintain organ specificity and promote their own maturation, successfully achieving blood perfusion, marking the first time that organoid blood vessels have acquired the ability of in vivo circulation.
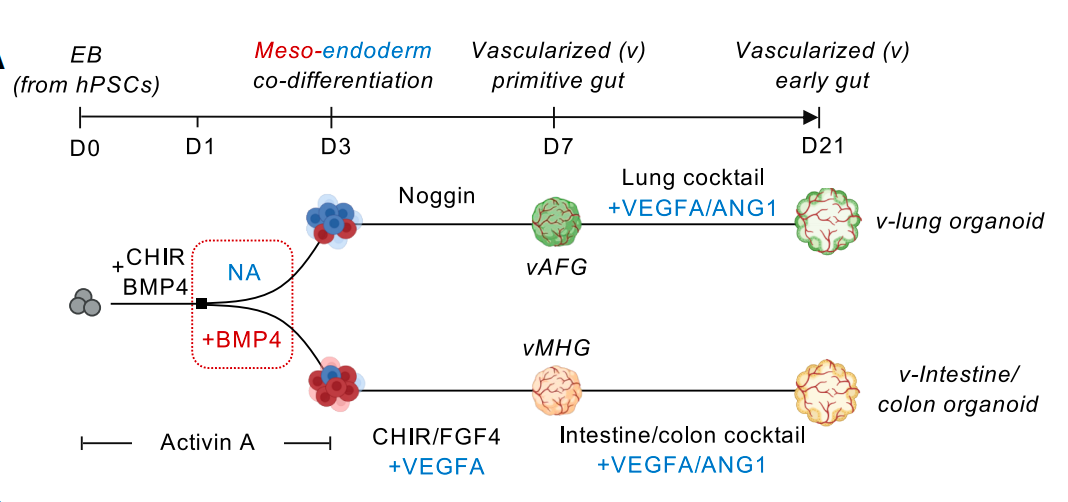
This study successfully constructed highly vascularized lung organoids and intestinal organoids for the first time through human induced pluripotent stem cells (IPscs). These organoid models not only simulate the complex process of multi-germ layer coordinated development in the early stage of human embryos, but also break through the bottleneck of traditional organoids lacking functional blood vessels and organ-specific mesenchymal matter, providing an advanced platform for studying complex intercellular communication in human organ development and diseases as well as regenerative medicine.
03 iPSC-NK
The iNK cell therapy derived from iPSC has become a new direction for researchers to explore the treatment of solid tumors, thanks to its multiple advantages such as nearly unlimited cell sources, efficient and stable CAR expression achieved with a single gene modification, high product uniformity, and more standardized production processes.
A recent study published in Science Advances, a sub-journal of Science, screened out LiPSC-GR1.1 as a high-quality iPSC cell line for optimizing iNK production. Through the genetic engineering modification of CAR targeting mesothelin (MSLN) and interleukin-15 (IL-15), the efficient differentiation of IPscs into mature activated iNK cells was successfully achieved. The obtained cells possess enhanced tumor-killing efficacy, excellent tumor homing ability and strong proliferation activity.
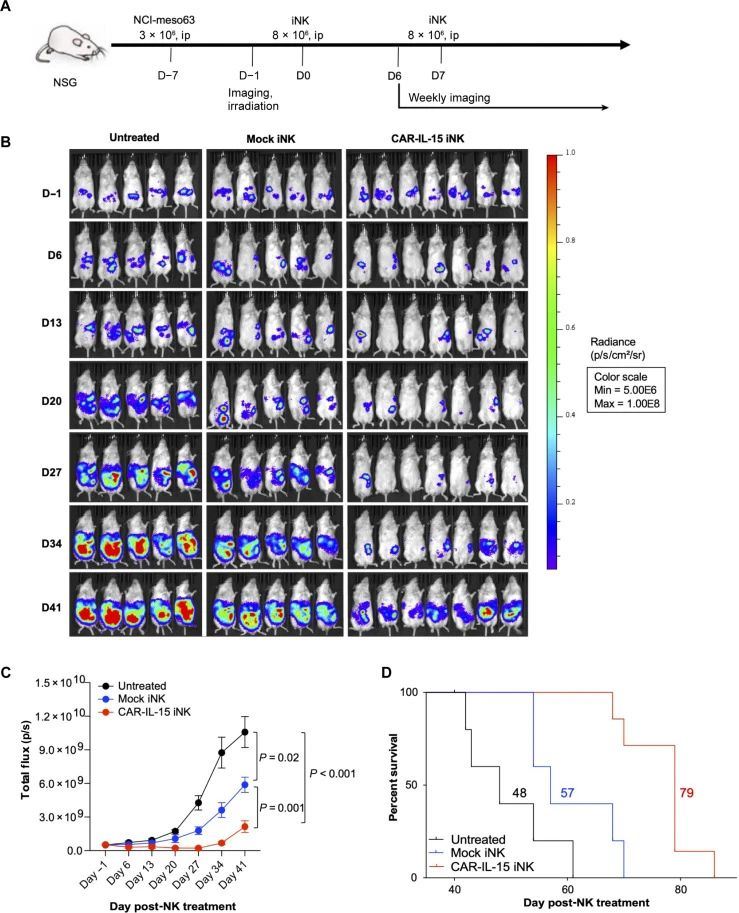
The anti-tumor efficacy of MSLN.CAR-IL-15 GR1.1-iNK cells in the NCI-meso63 mouse model
Studies show that, compared with mock iNK and MSLN.CAR iNK, MSLN.CAR-IL-15 iNK demonstrated the strongest killing ability against all the tested tumor cells (pancreatic cancer KLM-1, mesothelioma NCI-meso29, NCI-meso21, NCI-meso63, ovarian cancer OVCAR8 and gastric cancer cell line NCI-N87). Single-cell transcriptome analysis revealed that after treatment with CAR-IL-15 iNK, tumor cells that produce transforming growth factor -β (TGF-β) up-regulated the expression of major histocompatibility complex molecules and simultaneously down-regulated the expression of MSLN. Tumor-infiltrating CAR-IL-15 iNK cells exhibit a variety of characteristics conducive to tumor killing, enabling them to maintain an activated state, metabolic adaptability and effective tumor-killing ability in the complex tumor microenvironment.
This study indicates that IPSC-derived CAR-iNK has great potential in the treatment of solid tumors, providing a new solution for the treatment of solid tumors.
These breakthroughs in the fields of iPSC-CAR-NK cell therapy, IPSC-derived organoid vascular network construction, and IPSC-derived iNK cell therapy have not only achieved key breakthroughs in autoimmune diseases, organ development research, and solid tumor treatment, It has verified the diversified treatment concept of pluripotent stem cell therapy from "targeted clearance" to "regenerative repair", broken through the traditional technical bottleneck, established a new paradigm for the precise treatment of related diseases, and provided a more advanced platform and broader prospects for the in-depth exploration and clinical transformation of regenerative medicine, continuously promoting the medical field to move towards the goal of conquering more difficult and complicated diseases.