
In April 2024, the Drug examination Center of the State Drug Administration (CDE) accepted two types of stem cell-related drugs, including human umbilical cord mesenchymal stem cell injection and human amniotic mesenchymal stem cell injection.
Help Therapeutics Co., Ltd.
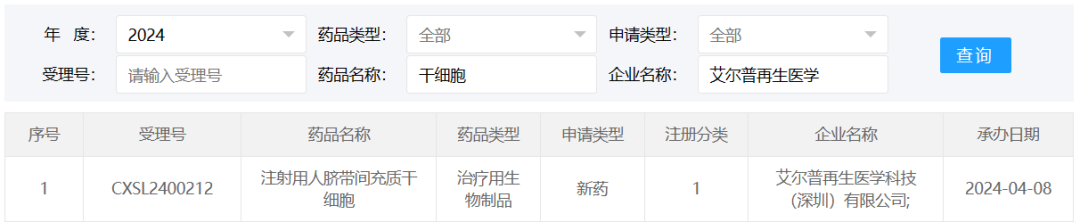
On April 8, 2024, the clinical trial application of "Human umbilical Cord Mesenchymal Stem cells for injection" developed by Help Therapeutics Co., Ltd. was accepted under the acceptance number CXSL2400212.
Help Therapeutics Co., Ltd. was founded in May 2016 and has built three multi-system intelligent platforms Help Cell-foundry: (1) a new generation of global iPSC reprogramming technology platform based on intelligent computing driven by diversified systems such as CRISPR/ chemical induction / non-animal origin; (2) intelligent cell quality inspection platform combined with machine learning methods such as semi-supervised learning; and (3) fully automated high-scale industrial cell intelligence platform.
This technology has been successfully selected into the National "13th five-year Plan" Scientific and technological Innovation Achievement Exhibition in 2021, and is in the forefront of the world in terms of cell mass production and quality assurance.
A number of R & D pipelines have been arranged around heart failure and tumor immunity and other diseases, among which iPSC technology therapy for the treatment of moderate and severe heart failure has entered a number of authoritative centers across the country to carry out large-scale randomized controlled human clinical trials.
Yuanpin Cell Biotechnology Group Co., Ltd.
On April 23, 2024, the clinical trial application of "Human amniotic Mesenchymal Stem Cell injection" issued by Yuanpin Cell Biotechnology Group Co., Ltd. was accepted under the acceptance number CXSL2400258.
Yuanpin is committed to tackling the key technology of amniotic mesenchymal stem cells in the treatment of ovarian dysfunction and has made some progress guided by clinical problems. previous studies have shown that human amniotic mesenchymal stem cells (hAMSCs) can effectively improve ovarian function in patients with early-onset ovarian insufficiency (POI). In December 2023, a study on the safety and efficacy of intravenous injection of hAMSCs in the treatment of early-onset ovarian insufficiency (POI) was published in Reproductive Sciences. Acute toxicity test, long-term toxicity test, tissue distribution test, hemolysis test and allergy test of intravenous injection of hAMSCs in the treatment of POI were carried out in different animal species to verify the safety and efficacy of hAMSCs intravenous injection in the treatment of POI model animals. It is expected to provide some reference for the intervention of POI patients in clinic.
Shenzhen Beike Bio-Technology Co., Ltd.
On April 28th, 2024, the "Human umbilical Cord Mesenchymal Stem Cell injection" independently developed by Shenzhen Beike Biotechnology Co., Ltd. obtained the implied approval (IND) of the new drug clinical trial from the Drug examination Center (CDE) of the State Drug Administration (CDE), the acceptance number is CXSL2400092, for the treatment of moderate and severe systemic lupus erythematosus. This innovation indicates that China's first mesenchymal stem cell drug for the treatment of moderate and severe systemic lupus erythematosus is about to enter a clinical trial.
Beike Biology is an early enterprise to introduce the concept of quality management in the field of cellular biomedicine. It carried out ISO9001 quality management system certification for the first time in 2010, and completed the three-in-one certification of "quality, environment, occupational health and safety" in 2012 and has passed the certification for 12 consecutive years. At the same time, the Jiangsu Branch of Beike Biology introduced and passed the comprehensive cell bank certified by the Association for the Development of Blood and Biotherapy (Association for theAdvancement of Blood & Biotherapies, AABB, formerly American Blood Bank Association) in Chinese mainland in 2012. In May 2017, the quality management system of four autologous cells (human umbilical cord-derived mesenchymal stem cells, human umbilical cord blood-derived hematopoietic stem cells, human placenta-derived mesenchymal stem cells and human peripheral blood immune cells) of Beike Biology Shenzhen Cell Bank (Shenzhen Comprehensive Cell Bank) was also certified by AABB.
As more and more new stem cell drugs enter the clinical stage, we expect stem cell therapy to be widely used in the future medical field.