
Source: doctor McKee
In July 2023, the Penn research team published an article entitled Ionizable Lipid Nanoparticles for Therapeutic Base Editing of Congenital Brain Disease to further study the applicability of LNP as a delivery carrier of gene editing platform in the perinatal brain for hereditary CNS (central nervous system disease), providing the possibility of brain editing for one-time single-gene disease treatment in perinatal or newborn babies.
Single gene genetic disease is one of the main causes of neonatal birth defects. at present, there are about 7000 to 10000 congenital diseases related to single gene mutations, of which about 17% involve the nervous system. including nerve growth retardation, motor and cognitive dysfunction, primary neuronal degeneration and other congenital brain diseases. Congenital diseases of the nervous system are not only lack of specific diagnostic indicators, but also lack of corresponding treatment methods, and often occur before the birth of the fetus, there is almost no possibility of reversal, so it is mainly to reduce symptoms or control the progression of the disease.
Gene editing technology helps in the treatment of single gene diseases
With the development of gene technology, the Nobel Prize-level gene editing technology CRISPR-Cas9 and base editing platform have emerged one after another, which makes scientists have new ideas for the thorough treatment of single-gene genetic diseases.Back in 2019, a team at the Children's Hospital of Philadelphia (CHOP) and the Pennsylvania School of Medicine used CRISPR gene editing technology to prevent a fatal lung disease in an animal model and published the findings on Science Translational Medicine.Although this is only a proof-of-concept study, the ability of gene editing to cure or alleviate diseases in the middle and third trimester of pregnancy and irreversible pathology is very exciting.Angel syndrome (Angelman syndrome) is a severe neurodevelopmental disorder that is currently incurable and has very limited treatment for babies who carry the defective mother's UBE3A gene.
In October 2020, a Nature research report showed that scientists using techniques such as CRISPR-Cas9 gene therapy to repair the wrong mutation of fetal brain in the womb and restore the function of UBE3A gene in human neuron culture can effectively or even permanently treat the defects of Angelman syndrome model.The researchers also demonstrated that this method has been shown to be effective in cultured human neurons.The results of this study not only provide an important basis for later scientists to treat Angelman syndrome, but also open the way for the treatment of other monogenic disorders.
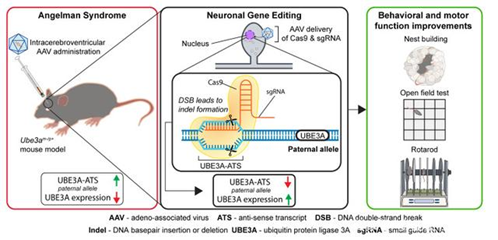 AAVMediate the knockout of UBE3A-ATS by CRSPR/Cas9 and restore the expression of UBE3 (photo source: DOI:10.1172/JCI142574)
AAVMediate the knockout of UBE3A-ATS by CRSPR/Cas9 and restore the expression of UBE3 (photo source: DOI:10.1172/JCI142574)
In Battle of Angels, the researchers used adeno-associated virus (AAV) gene therapy to transport Cas9 protein into the brains of embryonic mice with Angelman syndrome.Although the carrier system for delivering mRNA is effective in targeting the liver and heart in many proof-of-concept experiments, it is lack of effectiveness in targeting the central nervous system.Moreover, vector systems represented by AAV usually have limitations on the size of gene editing tools, especially in fetuses, the risks of viral immunity and vector integration need to be carefully considered.Non-viral vector delivery platform LNPs can achieve site-specific insertion, avoid random integration, so as to improve the safety of gene drugs; while targeted delivery of mRNA drugs, it can solve the problems of short-time degradation, repeated administration and high expression threshold requirements.As long as it is designed for the desired goals, the modular advantages of LNP can be brought into full play.
In 2021, the research team from Penn University developed a delivery system of intrauterine mRNA lipid nanoparticles (LNP). It was proved in pregnant mice that the selected LNP can safely deliver mRNA in uterus and improve the delivery effect of placental mRNA in vivo.This study lays a foundation for the development of new potential therapies for prenatal diagnosed genetic diseases, and verifies the feasibility of gene editing therapy for delivery of mRNA to the liver, lung and intestinal tract of perinatal mice by umbilical vein injection, but this study has not yet verified its delivery efficiency in the central nervous system.
LNP delivers mRNA to make a new breakthrough in gene editing in the brain
In July this year, after screening the LNP library in vivo to determine the best cell orientation of LNP in the brain of perinatal mice, the Penn research team optimized the formula of LNP in vitro, then used the optimized LNP to deliver thymine base editor (ABE) mRNA and synthesis guide RNA (sgRNA), and corrected the pathogenic single gene mutation of IDUA in mouse model by intracerebroventricular (ICV) injection.It successfully mediated the targeted gene editing therapy of LNP and the minimum immune response to the delivery vector.
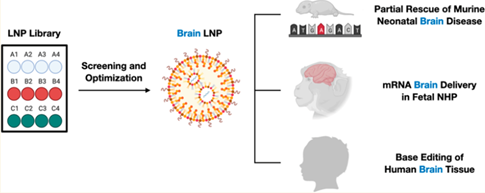
Guide map of experimental content
Under the comparison of the blank control group, the LNP vector designed by Penn's team not only had good targeting to the brain and had no significant expression in heart, lung, intestine, liver, spleen or kidney, but also similar distribution of LNP was observed in the whole brain: only in the ventricles, adjacent astrocytes and neurons with limited penetration into the internal structure of the brain.And there is no sign of spreading to the depth of the brain parenchyma outside the periventricular space.The researchers further optimized the parameters of C3 LNP, which performed best in the previous experiment. It was found that the best gene editing level could be obtained when the ratio of encapsulated mRNA to sgRNA was 3: 1, but when the N: P ratio was raised from 10:1 to 20: 1, the neuroblast delivery efficiency was better, but the toxicity was enhanced.
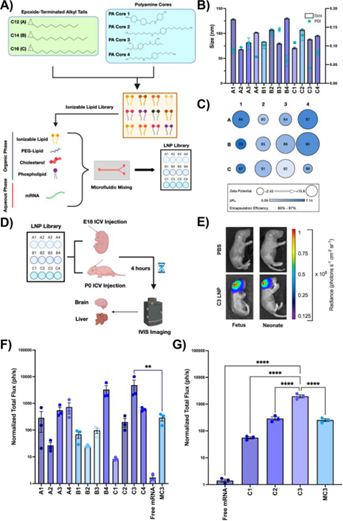
Design and Evaluation of Perinatal brain mRNA delivery LNP Library
In addition, the team further verified the transfection efficiency of the optimized LNP formula in crab eating monkey fetus and human cerebrospinal fluid (CSF). The results showed that the size and polydispersity of C3 LNPs did not change significantly, and the stability could be maintained for a week, while the transfection efficiency of C3 LNPs was high and had no significant effect on cell viability.The mRNA delivery efficiency of C3 LNPs in perinatal fetal brain can be transformed into larger animal models, and there is still room for further optimization.
Summary
Single-gene genetic disease, as a genetic disease controlled by only a pair of alleles, seems to change only one of the 20,000-30,000 human genes, but the result is that life is unbearable.For the research of gene therapy, there is not only a simple and accurate goal, but also a high requirement for the targeting and accuracy of editing technology.In July, 2023, the Penn research team also published mRNA-LNP-based hematopoietic stem cell gene editing therapy in Science, which overcomes the risk of in vitro gene editing hematopoietic stem cell therapy, makes gene editing therapy as simple as vaccination, and gives a glimmer of hope to more and more patients with rare diseases.
With the global sensation of the "gene editing baby" incident in 2018, gene editing technology has always been faced with questions about ethical issues.The unknown safety and uncontrollable consequences of "genetically modified" keep people in awe of the dignity of life and naturally reject overly radical gene therapy.
However, as long as we constantly improve technical norms and abide by the bottom line of ethics, and carry out research and progress in the framework of bioethics, rational use of gene editing can effectively solve some medical problems. Resolve some of the original crises beyond human control, and further explore the mysteries of life.Every child with rare disease is regarded as an "angel" sent by God to reveal genetic secrets. In the process of developing new drugs, especially for pregnant women, the safety of biological products used in fetal groups must be carefully examined and supervised to deepen the understanding of drugs and mechanisms without serious adverse reactions and effects.
It is believed that with the continuous development of gene editing technology and the continuous optimization of targeted delivery technology, gene editing therapy based on LNP delivery system will one day move from mouse experiment to clinical practice, and finally benefit those "angels" chosen by fate.
Reference:
1.https://doi.org/10.1021/acsnano.3c02268
2.https://doi.org/10.1021/jacs.2c12893
3.https://doi.org/10.1038/s41586-020-2835-2
4.https://doi.org/10.1126/science.adj0997