
On September 25, 2024, Deng Hongkui's research team of Peking University and Changping Laboratory, Shen Zhongyang and Wang Shusen's research team of Tianjin City First Central Hospital, and Hangzhou Ripu Chenchuang Technology Co., Ltd. collaborated to publish a research paper titled "Transplantation of Chemically Induced Pluritent Stem-Cell-Derived Islet Under Abdominal Anterior Rectus Sheath in a Type 1 Diabetes Patient."
This paper reports for the first time in the world a clinical study of using islet cell transplantation prepared from chemically reprogrammed induced pluripotent stem cells to treat type 1 diabetes. The first patient restored endogenous autonomous and physiological blood sugar control after transplantation, and completely and stably exited insulin injection treatment 75 days after transplantation. The efficacy has been stable for more than one year. This study initially proves that islet cell therapy prepared from chemically reprogrammed pluripotent stem cells is safe and effective, achieving clinical functional cure of type 1 diabetes.

01 Diabetes
Our country has become the country with the largest number of diabetes patients in the world.
Diabetes is one of the major diseases threatening human health on a global scale, and our country has become the country with the largest number of diabetes patients in the world. Currently commonly used treatment methods, such as insulin injections and anti-diabetic drugs, are difficult to achieve precise control of blood sugar, resulting in multiple complications, seriously affecting patients 'quality of life, and even endangering lives. After more than 40 years of clinical accumulation, islet transplantation has achieved good clinical results in the treatment of diabetes. However, the shortage of human pancreatic donors has seriously limited its widespread application.
02 Diabetes stem cell treatment
Islet cells prepared from human pluripotent stem cells provide a new source for diabetes transplant treatment.
Islet cells prepared from human pluripotent stem cells provide a new source for diabetes transplant treatment. Pluripotent stem cells have the characteristics of unlimited proliferation and the ability to differentiate into all functional cell types of organisms. They have wide range of important application values in cell therapy, drug screening and disease models. They are the most potential "seed cells" in the field of regenerative medicine.
In 2006, Japanese scientist Shinya Yamanaka reported the use of genetically modified methods to reprogram adult cells into pluripotent stem cells, called induced pluripotent stem cells (iPS cells). iPS technology provides a new method for constructing patient-specific stem cell lines and won the 2012 Nobel Prize in Physiology or Medicine. At present, clinical research on cell therapy with functional cells prepared through iPS technology has increased year by year. However, so far, there has been no report that this technology has achieved clinical breakthroughs in curing major diseases. In addition, iPS cells prepared through the transgenic overexpression pathway pose potential safety risks and cannot accurately control the extent and effect of transgenic reprogramming.

In 2013, Deng Hongkui's research team published an original result in the journal Science. Using only exogenous small chemical molecules, cell fate can be reversed (called chemical reprogramming) and mouse somatic cells can be reprogrammed into pluripotent stem cells (Chemically induced pluripotent stem cells, CiPS cells). On this basis, the use of small chemical molecules to induce human adult cells into pluripotent stem cells (human CiPS cells) was further realized. The establishment of this technology opened up a new way for the preparation of human pluripotent stem cells, which is useful for cell therapy and artificial organs provide a more ideal source of cells.
After more than 20 years of systematic research, Deng Hongkui's team took diabetes treatment as the entry point and based on the key core technology of chemical reprogramming, established a plan for human CiPS cells to efficiently differentiate functionally mature islets, and tested it in experimental animal models of primate diabetes. In vivo, it proved the feasibility, safety and effectiveness of human CiPS cells to differentiate into islet cells for the treatment of diabetes. On this basis, a new islet transplantation strategy,"anterior subsheath transplantation of rectus abdominis", was further established to achieve long-term survival and functional maintenance of islets after transplantation. These preclinical studies have laid a solid foundation for subsequent clinical research.
In June 2023, the research team was officially approved for the national stem cell clinical research filing (filing number: MR-12-23-017130), and carried out exploratory clinical research on the transplantation of human CiPS cell-derived islet cells for the treatment of type 1 diabetes.
03 Research process

This study used islets prepared from human CiPS cells to achieve clinical functional cure of diabetes.
The first patient enrolled in the study was a patient with type 1 diabetes with a history of 11 years. His blood sugar could not be effectively controlled with intensive insulin therapy. In order to treat diabetes, the patient underwent a pancreatic transplant in May 2017, but one year later the pancreatic transplant failed and the patient showed strong positive group-reactive antibodies, making it difficult to accept allogeneic islet transplantation. However, autologous cell transplantation may solve this problem. In this clinical study, before transplantation, the proportion of time patients 'blood sugar was within the target blood sugar range was only 43.18%. Severe hypoglycemia occurred many times in the past year, seriously threatening the patient's life safety.
On June 25, 2023, the patient received autologous human CiPS cell-differentiated islet transplantation. The patient returned to normal activities on the day after transplantation, and the islet cells survived well. The fasting C-peptide increased from <0.02 ng/ml before transplantation to 0.31 ng/ml after transplantation. On the 10th day after transplantation, the patient's blood sugar was stable, and the daily insulin requirement has been reduced to half of that before transplantation.
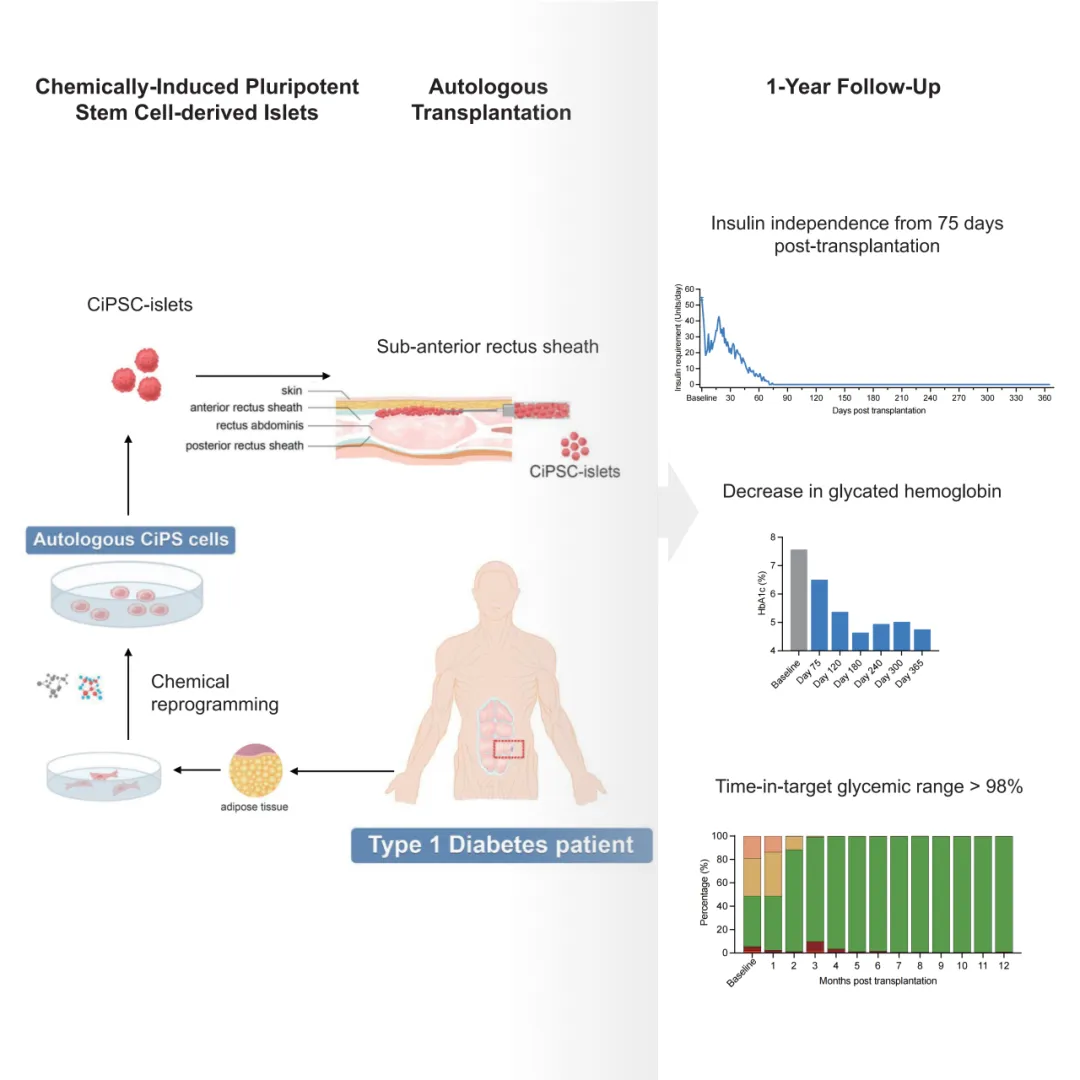
Schematic diagram and efficacy display of clinical studies based on CiPS in the treatment of type 1 diabetes
The study reported data on the clinical endpoints of effectiveness and safety for this patient's clinical treatment for 1 year. On the basis of demonstrating safety, the study obtained key data on clinical effectiveness:
1)After transplantation, the patient's fasting blood sugar level gradually returned to normal, and the need for exogenous insulin continued to decrease. Starting from the 75th day after transplantation, the patient was completely and stably released from insulin injection therapy. As of the time of publication of the paper, the patient had completely withdrawn from insulin therapy for more than 1 year;
2)Glycosylated hemoglobin levels dropped to 4.76% one year after transplantation;
3)The patient's blood sugar compliance rate continued to increase from the baseline value of 43.18%, exceeded 98% 5 months after transplantation and remained at this level. These results demonstrate that this therapy achieves clinical functional cure of type 1 diabetes.
A key issue that needs to be solved in pluripotent stem cell technology treatment is how to ensure the safety and controllability of transplanted cells in vivo. The research team innovatively transplanted islets into the subsheath of the anterior rectus abdominis. Compared with the traditional islets transplantation strategy, this transplantation strategy is less invasive, simple to operate, easy to track and observe the graft for long-term, and can be removed if necessary. This study achieved effective monitoring of grafts through ultrasound and nuclear magnetic means for the first time in clinical practice, greatly improving the safety and controllability of the stem cell clinical treatment research process.
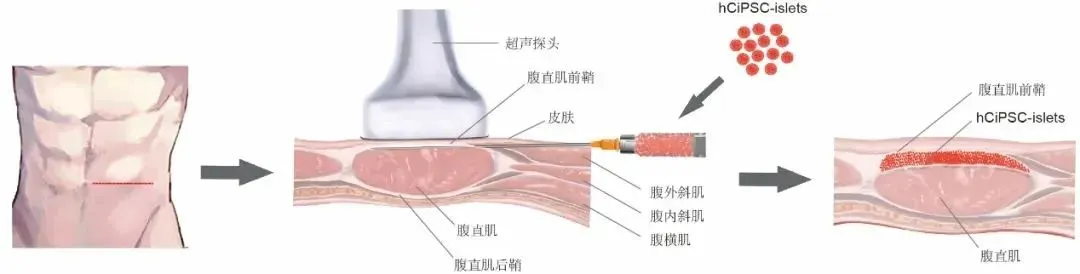
Schematic diagram of transplantation of islet cells under the anterior sheath of the rectus abdominis
This study used islets prepared from human CiPS cells to establish a new cell therapy approach for diabetes and achieve clinical functional cure of diabetes. More importantly, the success of functional cells prepared by chemical reprogramming technology in clinical treatment of diseases shows that chemical reprogramming is expected to become a universal underlying technology for efficiently preparing various functional cell types, and is widely used in cell therapy in the treatment of major diseases. Applications have opened up a new path.